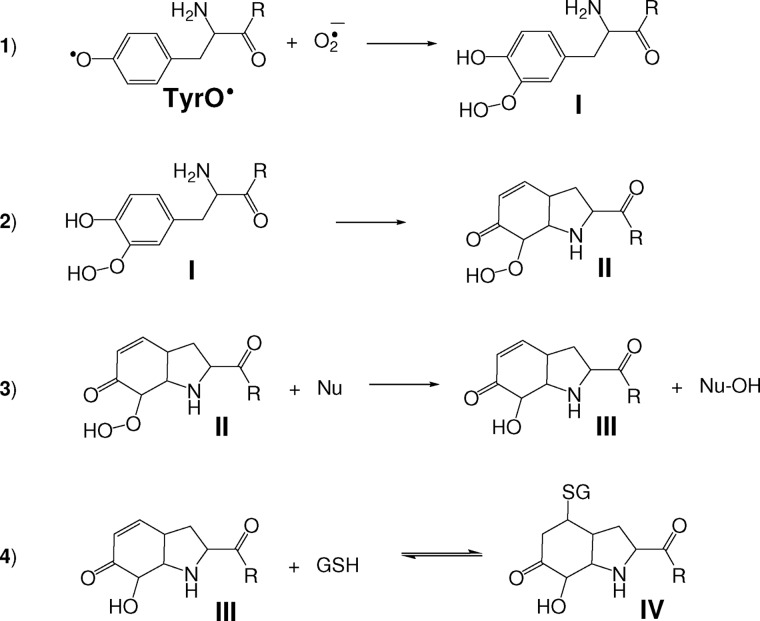
SCHEME 1.
Proposed mechanism for the formation of the Tyr-hydroxide-GSH adduct. 1, the addition of superoxide to Tyr radicals results in the formation of hydroperoxide species (I). The scheme shows the addition at the ortho position but it could also occur at the para position. 2, Michael addition of the Tyr amine (or amide) nitrogen to the Tyr ring gives a bicyclic hydroperoxide derivative (II). 3, reduction of the hydroperoxide species by a nucleophile (Nu) gives the corresponding alcohol (III; the Tyr-hydroxide species was characterized for the parent Tyr amino acid as 3a-hydroxy-6-oxo-2,3,3a,6,7,7a-hexahydro-1H-indol-2-carboxylic acid or 4-alanyl-4-hydroxy-cyclohexadienone for superoxide addition to the ortho positions; see Nagy et al. (10). 4, Michael addition of GSH to the α-β unsaturated carbonyl group of III gives IV.