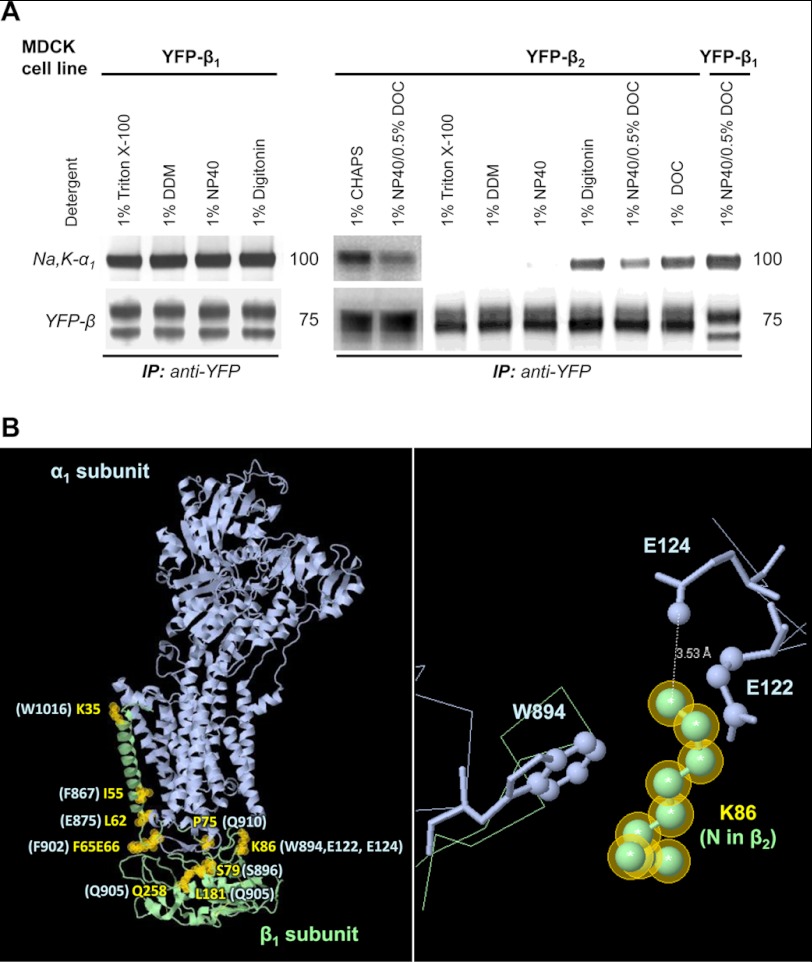
FIGURE 7.
The Na,K-ATPase α1-β1 complex is more stable than the Na,K-ATPase α1-β2 complex in detergent extracts from MDCK cells. A, MDCK cells stably expressing either YFP-β1 or YFP-β2 were lysed by incubation with the extraction buffer containing an appropriate detergent (as indicated). After scraping the cells and removing non-extracted material by centrifugation, YFP-linked β1 or β2 subunits were immunoprecipitated. Immunoprecipitated YFP-β1 or YFP-β2 and co-immunoprecipitated α1 subunit were analyzed by Western blot. Co-immunoprecipitation of the Na,K-ATPase α1 subunit with YFP-β1 was detected in all tested detergents. In contrast, co-immunoprecipitation of the Na,K-ATPase α1 subunit with YFP-β2 was observed only in selected detergents. B, a model of the Na,K-ATPase α1 and β1 subunits based on the crystal structure of the sodium-potassium pump at 2.4 A resolution (Protein Data Bank code 2ZXE) (39) shows the α1-interacting residues in the β1 subunit that are different in the β2 subunit (yellow labels and yellow shaded circles). The corresponding β1-interacting residues in the α1 subunit are indicated in light blue. A close-up view of Lys-86 of the β1 subunit and its interacting residues in the α1 subunit is shown in the right panel.