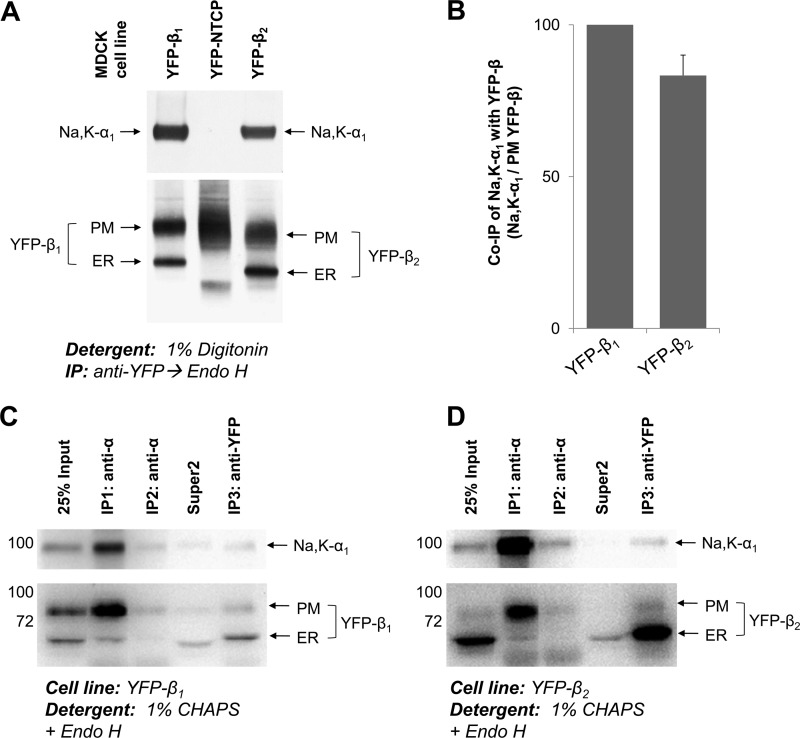
FIGURE 8.
The Na,K-ATPase α1-β2 complex is preserved in digitonin and CHAPS extracts from MDCK cells. A, proteins were extracted from MDCK cells expressing YFP-β1, or YFP-β2, or YFP-linked bile acid transporter (YFP-NTCP), with the extraction buffer containing 1% digitonin. YFP-linked β1 or β2 subunits were immunoprecipitated and treated with Endo H followed by elution of proteins from the beads. Co-immunoprecipitation of the Na,K-ATPase α1 subunit was detected with YFP-β1 and with YFP-β2, but not with YFP-NTCP, indicating that there is no nonspecific precipitation of the α1 subunit. B, densitometric quantification of the results presented in A was performed by dividing the density of the α1 subunit band by the density of the corresponding PM YFP-β band. C and D, proteins were extracted from MDCK cells expressing either YFP-β1 (C) or YFP-β2 (D) using extraction buffer containing 1% CHAPS and subject to successive rounds of immunoprecipitation (IP) using anti-α antibody followed by a final round of immunoprecipitation using anti-GFP/YFP antibody. Both extracted and immunoprecipitated proteins were treated with Endo H prior to SDS-PAGE. The majority of YFP-β subunits co-immunoprecipitated with α1 were complex-type glycosylated. Almost all of the β subunits not assembled with α1 were Endo H-sensitive and immunoprecipitated in the final anti-GFP/YFP immunoprecipitation. PM, plasma membrane; Super2, supernatant after the second round of immunoprecipitation.