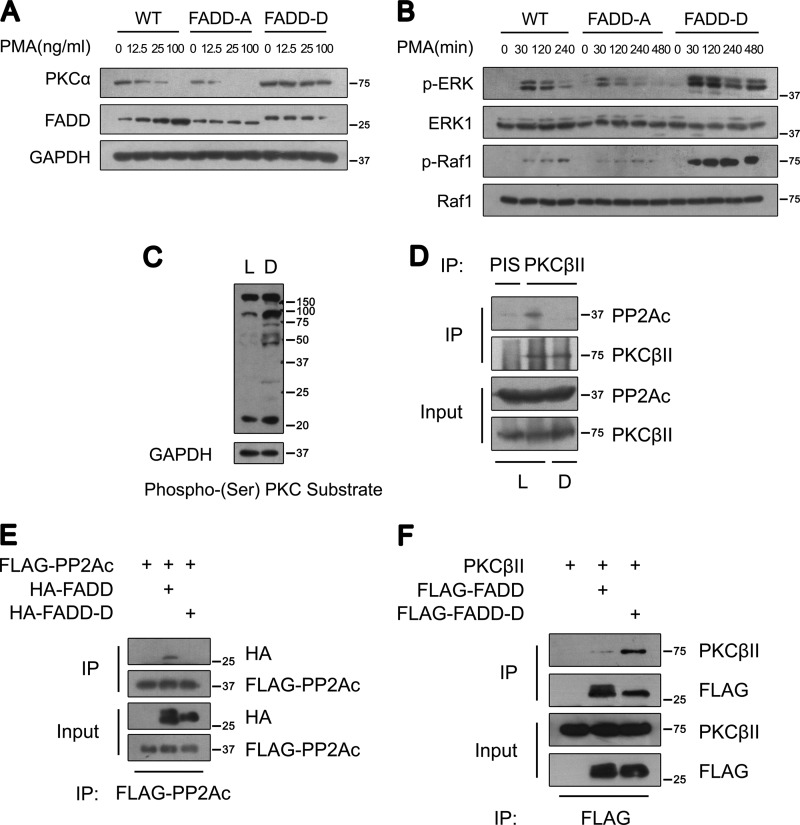
FIGURE 6.
Ser-191 phosphorylation of FADD regulates cPKC phosphorylation, stability, and signaling. A, Western blot analysis of PKCα in WT, FADD-A, and FADD-D MEFs. MEFs were treated with PMA at the indicated concentrations for 12 h. B, Western blot analysis of phosphorylated ERK and Raf-1 in WT, FADD-A, and FADD-D MEFs. MEFs were treated with 100 ng/ml PMA for the times indicated. C, Western blot analysis of phospho-(Ser) PKC substrates in cytosolic extracts of cardiac muscles from control littermates (L) and FADD-D mice (D). Representative results from five pairs of mice are shown. D, coimmunoprecipitation (IP) analysis of endogenous PKCβII and PP2Ac from cardiac muscles of control littermates (L) and FADD-D mice (D). Representative results from three pairs of mice are shown. E, coimmunoprecipitation analysis of FADD and PP2Ac in 293T cells. As indicated, lysates from the cells transfected with plasmids encoding FLAG-PP2Ac, HA-FADD, or HA-FADD-D were subjected to immunoprecipitation with an anti-FLAG antibody. FADD and FADD-D were detected using an anti-HA antibody. F, coimmunoprecipitation analysis of FADD and PKCβII in 293T cells. As indicated, lysates from the cells transfected with plasmids encoding FLAG-FADD, FLAG-FADD-D, or pcDNA-PKCβII were subjected to immunoprecipitation with an anti-FLAG antibody. PKCβII was visualized by anti-PKCβII.