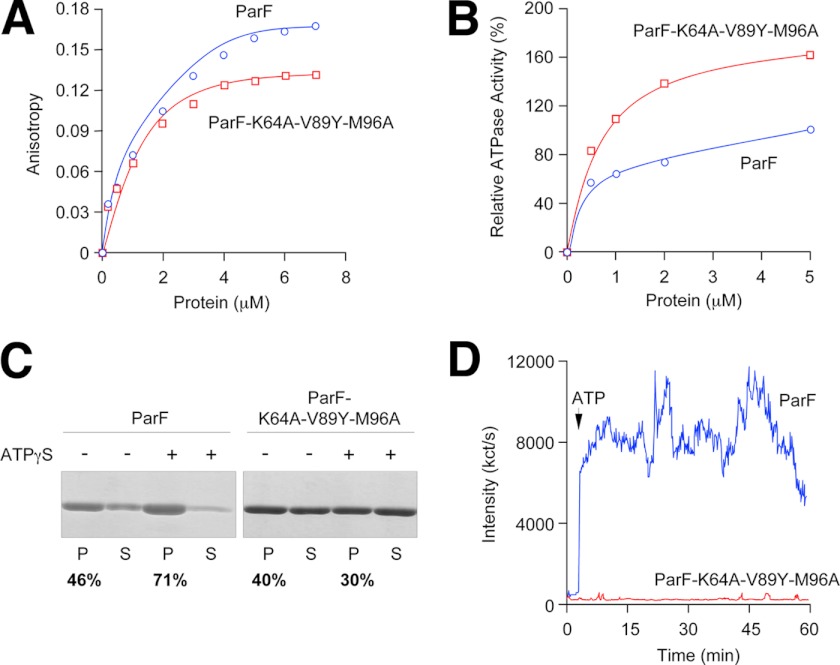
FIGURE 5.
Biochemical and polymerization properties of wild-type ParF and the ParF(K64A/V89Y/M96A) mutant. A, anisotropy changes when MANT-ATP (1 μm) was titrated with increasing concentrations of wild-type ParF or ParF(K64A/V89Y/M96A). B, ATPase activity of wild-type ParF and ParF(K64A/V89Y/M96A) incubated with ATP (250 μm). Activities are expressed relative to the level of ATP hydrolysis observed with the wild-type protein at 5 μm. C, sedimentation assays in which wild-type ParF and ParF(K64A/V89Y/M96A) (6–8 μm) were incubated in the absence (−) or presence (+) of nucleotides (2 mm). The pellet (P) and supernatant (S) fractions were resolved on a 12% SDS gel and stained with Coomassie Blue. The percentages of ParF proteins detected in pellet fractions are shown. D, polymerization of wild-type ParF and ParF(K64A/V89Y/M96A) proteins monitored by DLS. Proteins (2.16 μm) were incubated at 30 °C for 3 min, at which time ATP (500 μm; arrow) and MgCl2 (5 mm) were added. Reactions were followed for a further ∼57 min. Data in A–D are representative examples of experiments performed at least in duplicate.