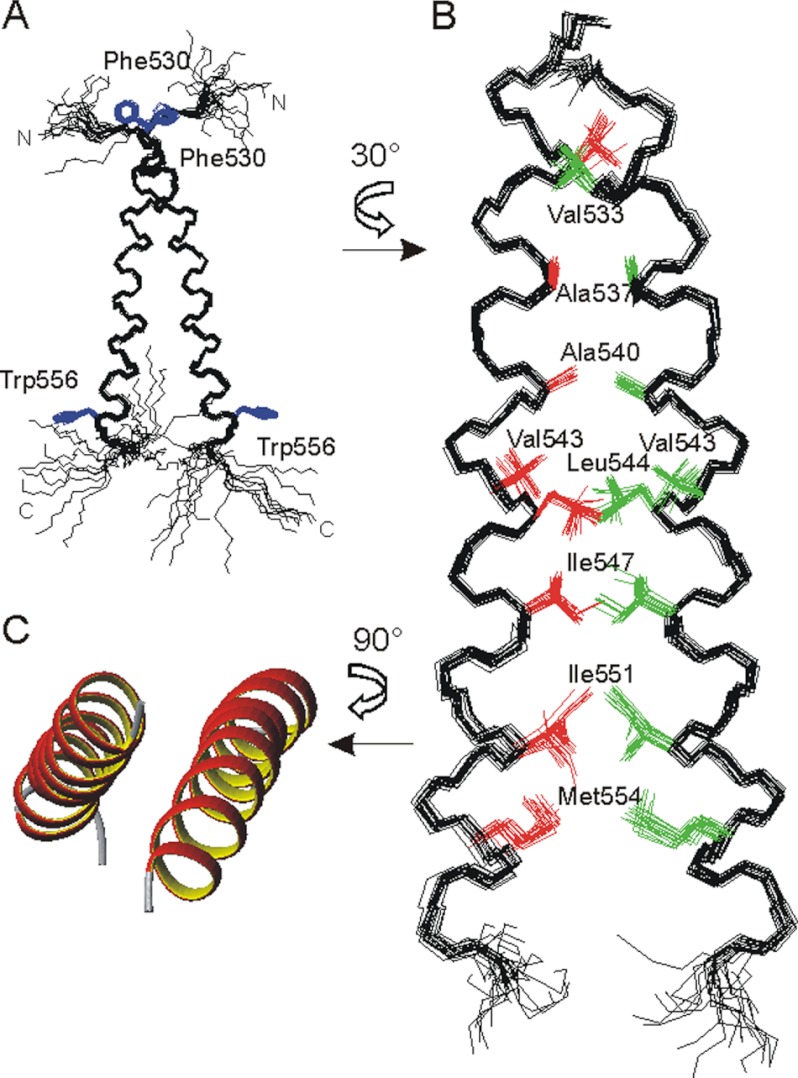
FIGURE 1.
A and B, superposition of the 10 best NMR structures of the PDGFRβ-TM dimer (amino acids 526–563). A, backbone representation, with disordered N and C termini, illustrating the dimer crossing angle of ∼20°. The side chains of Phe-530 and Trp-556 are shown in blue for visual orientation. B, details of the interface after a rotation of 30° around the z axis. The contributing residues are depicted in red and green, and the disordered termini are removed for clarity. C, ribbon representation of the helical regions after rotation of the dimer by 90° around the x axis.