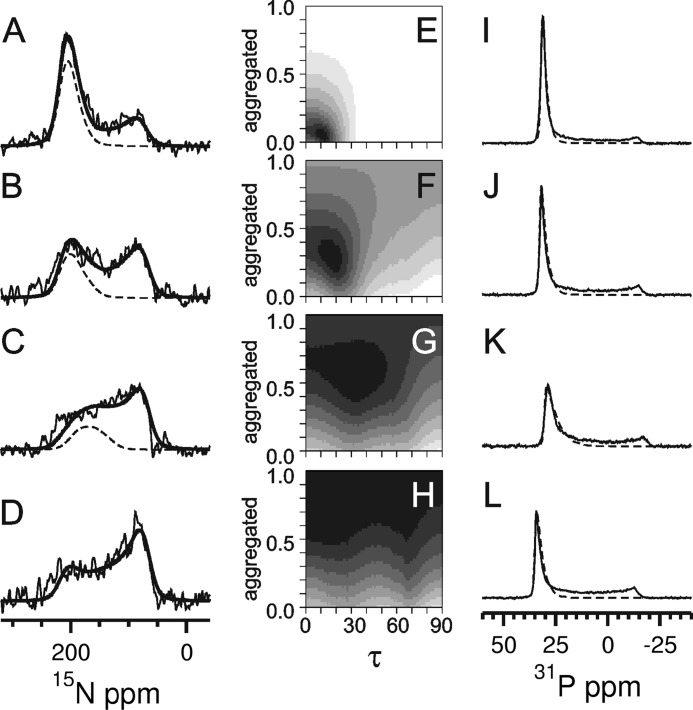
FIGURE 2.
Solid state 15N NMR (A–D) and 31P NMR spectra (I–L) of PDGFRβ-TM in macroscopically oriented bilayers in the liquid crystalline state. The peptide was reconstituted in lipids of different thickness, namely in DEPC (A and I), POPC (B and J), DMPC (C and K), and DLPC (D and L) at a peptide:lipid ratio of 1:200. The 15N NMR spectra were fitted by a sum of three contributions: (i) well aligned “oriented” peptides with a transmembrane orientation (resonances between ∼220 and 170 ppm depending on the local tilt angle τ), (ii) reconstituted transmembrane peptides that are present in “nonoriented” parts of the bilayer sample, plus (iii) “aggregated” peptides that were not properly reconstituted (for the latter contributions (ii) and (iii): resonances between 220 and 60 ppm. The sum of all three contributions is shown as a thick solid line, and the contribution of the well oriented peptide (i) as a dashed line. The fraction of properly reconstituted yet nonoriented peptides (ii) was determined from the 31P NMR spectra (I–L) of the phospholipids. These lineshapes were fitted by a sum of two contributions of oriented membranes (dashed line) plus nonoriented membranes. The 15N NMR spectra (A–D) were simulated for varying tilt angles τ and with a varying fraction of aggregated peptides, to be compared with the experimental lineshapes in the form of root mean square deviation maps (E–H). These maps are thus displayed as a function of the tilt angle τ and the fraction of aggregated peptide. Neighboring root mean square difference steps differ by a factor of 1.2, showing the best fit area in black. The 15N NMR spectrum of DLPC (D) could not be reliably deconvoluted into the different contributions due to excessive aggregation. In the other lipid environments, a decrease in helix tilt angle and in the aggregated fraction was observed for increasing bilayer thickness (E–G).