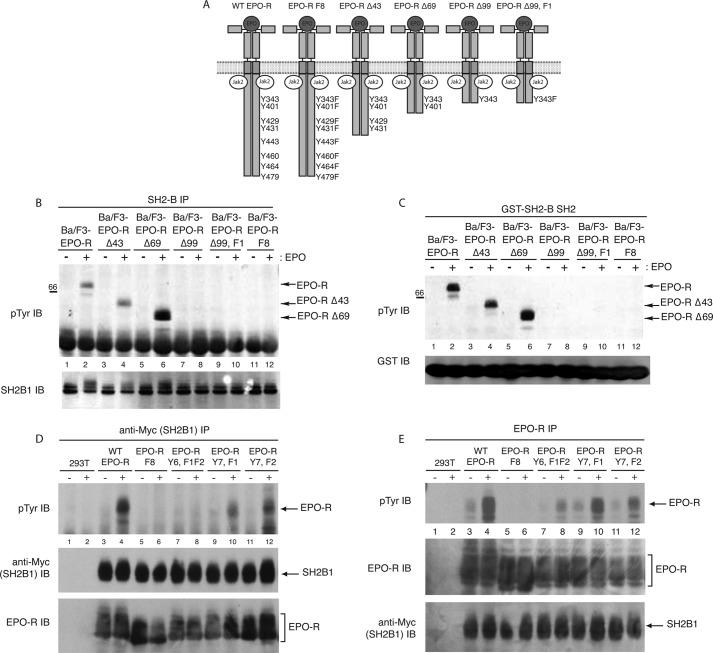
FIGURE 3.
Co-immunoprecipitation with EPO-R truncation mutants confirms SH2B1 binds specifically to Tyr(P)-343 and Tyr(P)-401 of the EPO-R. A, panel of EPO-R truncation mutants used. Deletion mutant numbers correspond to the number of amino acids deleted from the cytoplasmic tail of the EPO-R. B, SH2B1 immunoprecipitations (IP) were performed on lysates from EPO-stimulated and -unstimulated Ba/F3 cells expressing the various EPO-R mutants, followed by phosphotyrosine immunoblotting (IB). The membrane was reprobed with anti-SH2B1. C, GST-SH2B1 SH2 pulldowns were performed on lysates collected from EPO-stimulated and -unstimulated Ba/F3 cells expressing the EPO-R truncation mutant panel. Pulled down complexes were analyzed via phosphotyrosine immunoblot. Lysates were probed with anti-GST. D, 293T cells were transfected to express Myc-tagged SH2B1 and the indicated EPO-R tyrosine mutants. Transfected cells were depleted of cytokines and either left unstimulated or stimulated with 5 units/ml EPO for 10 min. Immunoprecipitation was performed with an anti-Myc antibody, and the membranes were probed with anti-Tyr(P) and reprobed with anti-EPO-R and anti-Myc (to detect myc-SH2B1β). E, from identical lysates as shown in D, immunoprecipitations were performed with an EPO-R antibody, followed by phosphotyrosine immunoblotting and reprobing with anti-EPO-R and anti-Myc.