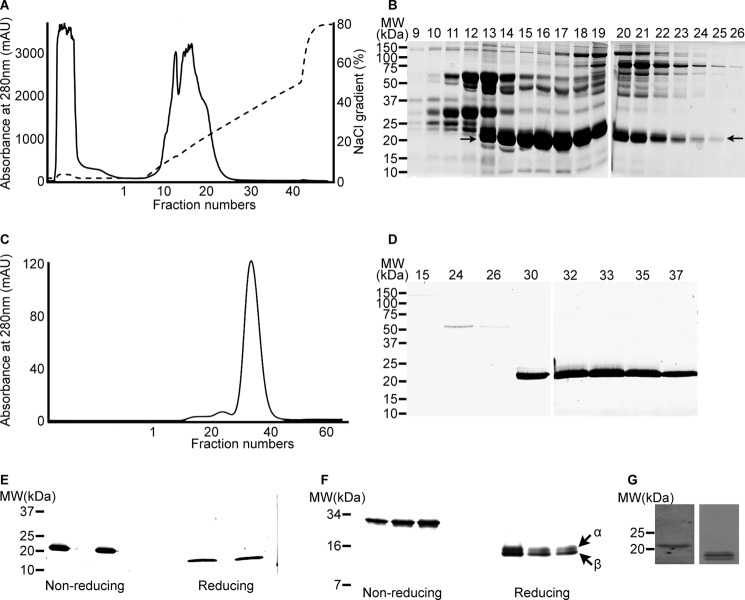
FIGURE 1.
Purification of 21-kDa protein. 10 mg of venom was separated by Q-Sepharose ion-exchange chromatography (A), and the selected fractions (9–26) were analyzed by 10% non-reducing SDS-PAGE (B). The partially purified protein at 21 kDa is indicated by arrows. Selected fractions were further separated by Superdex 75 gel filtration chromatography (C) and analyzed by 10% non-reducing SDS-PAGE (D). The purified venom protein was analyzed under non-reducing and reducing conditions by 4–20% gradient SDS-PAGE (E) and 10–20% Tris-Tricine gels (F). The cross-reactivity of antibody raised against the snaclecs of E. ocellatus with the purified protein was analyzed by immunoblot (obtained from a Tris-Tricine gel under non-reducing (left) and reducing (right) conditions) (G). mAU, milliabsorbance units; MW, molecular weight.