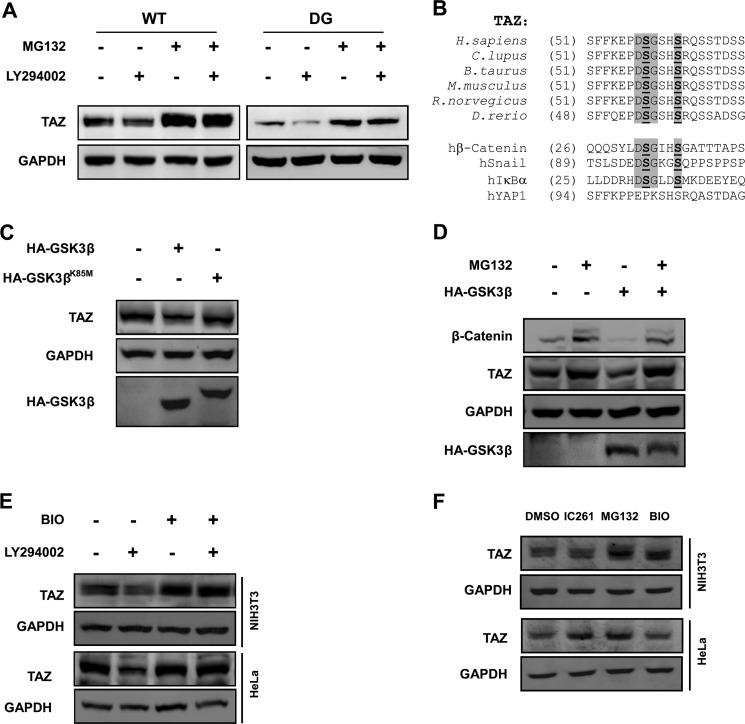
FIGURE 3.
GSK3 destabilizes TAZ. A, C-terminal phosphodegron is not responsible for LY294002-induced TAZ degradation. NIH3T3 cells stably expressing TAZWT and TAZDG (C-terminal phosphodegron DG mutant) were treated with or without LY294002 and MG132, as indicated. TAZ protein levels were determined by WB along with the GAPDH control. B, alignment of N-terminal phosphodegron in TAZ from different organisms. The phosphodegrons of β-catenin, Snail, and hIκBα are included for comparison with the DSGXXS motif. C, kinase activity of GSK3β is required for TAZ degradation. The WT or kinase-dead K85M mutant of GSK3β was transfected into HeLa cells, and endogenous TAZ protein levels were determined. D, MG132 blocks GSK3β overexpression-induced decrease of TAZ protein level. HA-GSK3β was transfected into HeLa cells as indicated, followed by treatment with MG132 (10 μm) for 6 h as indicated. Endogenous TAZ protein levels were determined along with the GAPDH control. β-Catenin was included as a positive control. E, PI3K inhibitor-induced TAZ degradation is blocked by GSK-3 inhibitor. HeLa and NIH3T3 cells were treated with PI3K inhibitor (LY294002) or GSK3 inhibitor (BIO) as indicated for 6 h. Endogenous TAZ protein levels were determined by WB along with the GAPDH control. F, regulation of TAZ protein levels in a cell type-dependent manner. HeLa and NIH3T3 cells were treated with casein kinase 1δ/1ϵ inhibitor (IC261), MG132, and GSK-3 inhibitor (BIO) as indicated, and endogenous TAZ protein levels were determined by WB along with the GAPDH control.