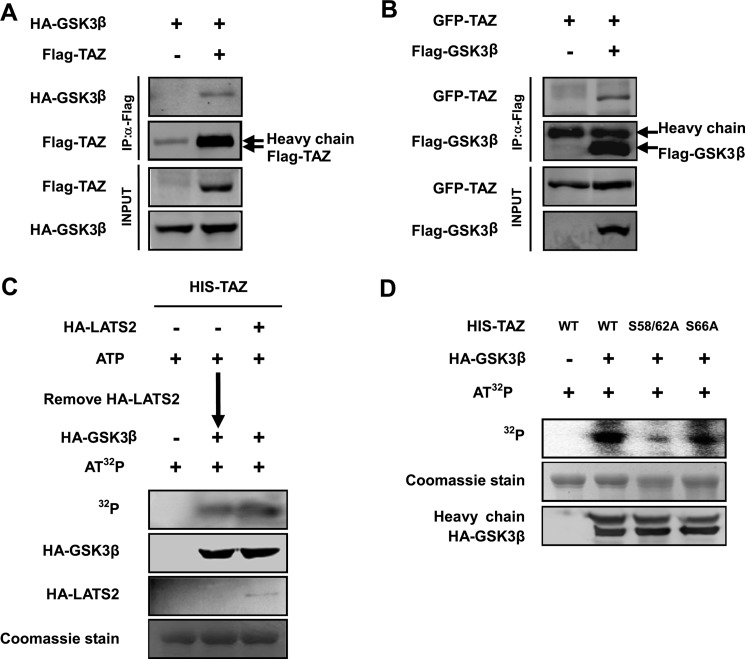
FIGURE 4.
GSK3 interacts with and phosphorylates TAZ. A and B, TAZ binds to GSK3β. GSK3β was co-transfected with TAZ into HEK293T cells as indicated. GSK3β and TAZ associations were examined by reciprocal co-immunoprecipitation (IP) as indicated. C and D, GSK3β directly phosphorylates the TAZ N-terminal phosphodegron in vitro. His-TAZ WT or mutants were expressed and purified from E. coli. HA-LATS2 and HA-GSK3β were immunoprecipitated from transfected 293T cells. In vitro kinase assay was performed using purified His-TAZ as a substrate. C, the purified TAZ was incubated with HA-LATS2 immunoprecipitated from transfected HEK293T cells in the presence of cold ATP. HA-LATS2 was removed from the kinase reaction. The LATS-treated His-TAZ was then incubated with HA-GSK3 immunoprecipitated from transfected HEK293T cells in the presence of radioactive ATP. D, phosphorylation of His-TAZ WT, S58A/S62A, and S66A mutants was also performed by adding GSK3β without the LATS2 pretreatment. Phosphorylation of TAZ was detected by a 32P autoradiograph. His-TAZ used in the in vitro reaction was shown by Coomassie Blue staining.