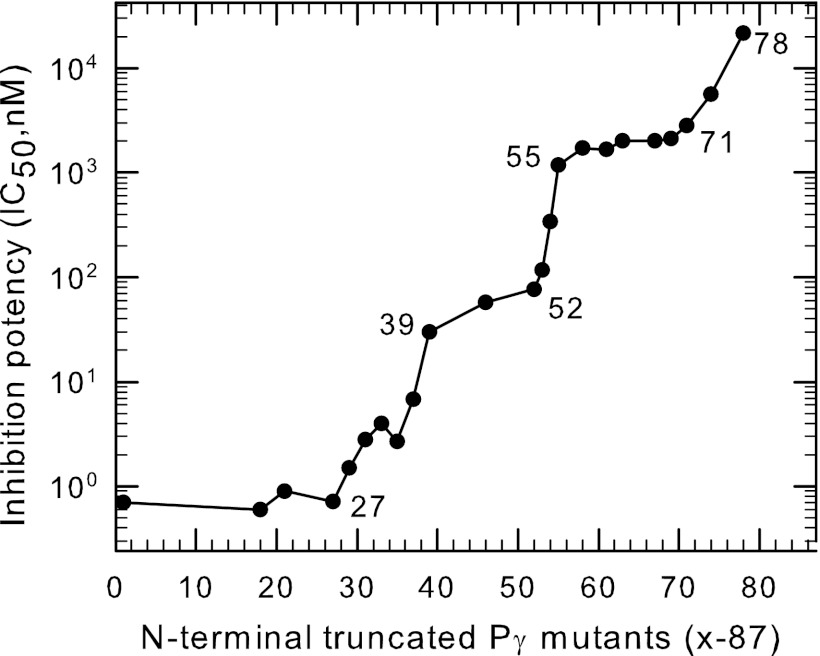
FIGURE 1.
Multiple regions of Pγ stabilize its interaction with PDE6 catalytic dimer to inhibit catalysis. Purified Pαβ (0.2 nm) was pre-incubated with the indicated N-terminal truncated Pγ mutants (Pγx-87) for 20 min, followed by addition of 2 mm cGMP substrate. Catalytic activity was measured by the phosphate release assay. The inhibition potency (IC50) was calculated from curve fitting the results to a 3-parameter logistic equation. The data represent the mean of at least three experiments; error bars (coefficient of variation < 10% in all cases) were omitted for clarity. The abscissa represents the position number of the starting amino acid of the N-terminal truncated Pγ mutant, with position 1 being the wild-type sequence. Data for Pγ63–87, Pγ71–87, Pγ74–87, and Pγ78–87 were taken from Ref. 10.