FIGURE 2.
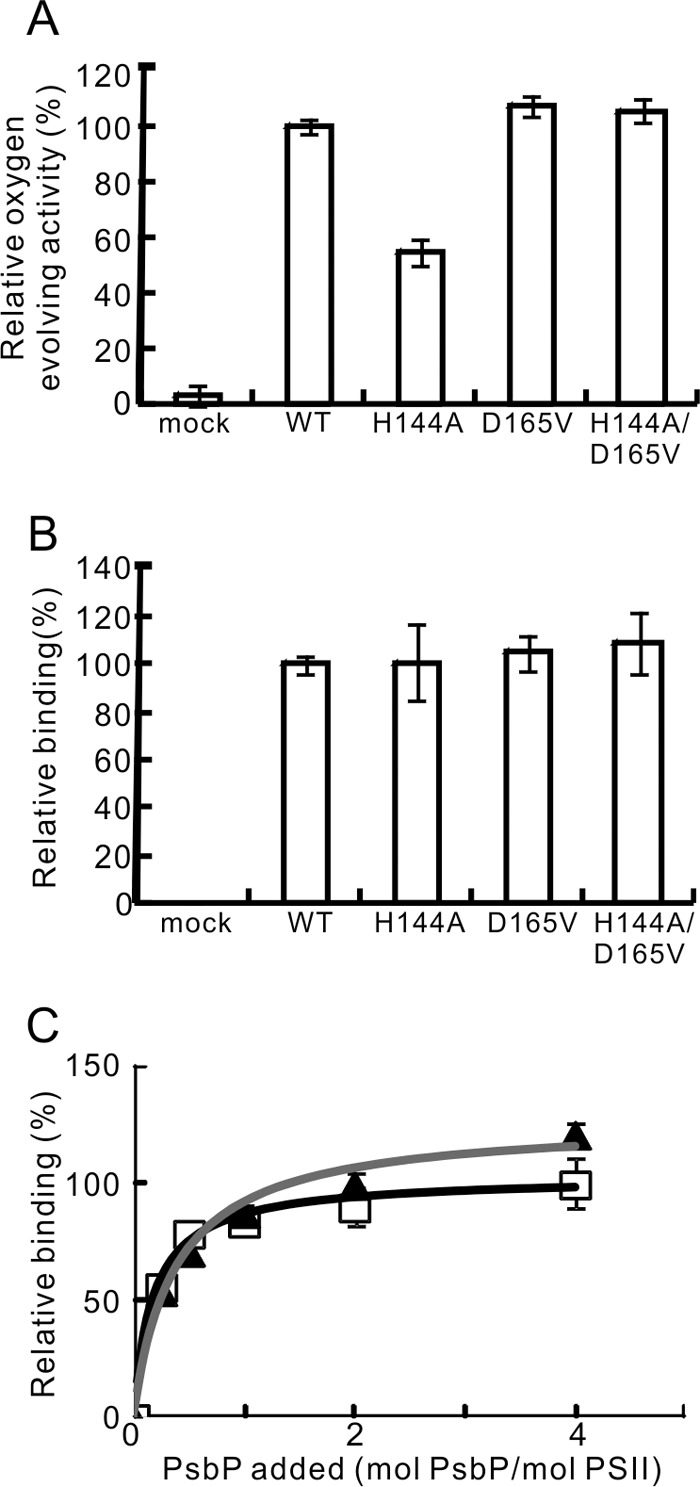
The oxygen-evolving activity of PSII reconstituted with WT, H144A, D165V, and H144A/D165V respectively, and the binding ability of these PsbP proteins to PSII. A, oxygen-evolving activity was measured in the absence of Ca2+ and Cl− ions. WT-reconstituted PSII activity (216 μmol O2/mg Chl/h) was set at 100%. n = 3, error bars = S.D. B, the quantities of the PSII-bound PsbP following reconstitution were quantified from the intensity of the fluorescence in the SDS-PAGE gel. The band intensity in the mock lane was subtracted from the intensity of each test lane. The intensity of the WT was set at 100%; n = 3, error bars = S.D. C, binding profiles of WT (squares, black line) and H144A (triangles, gray line) to NaCl-washed PSII. PSII was reconstituted with WT or H144A in various molecular PsbP:PSII ratios, and the amount of PSII-bound PsbP was quantified as in B. The intensity of WT:PSII = 4:1 was set at 100%; n = 4, error bars = S.D.
