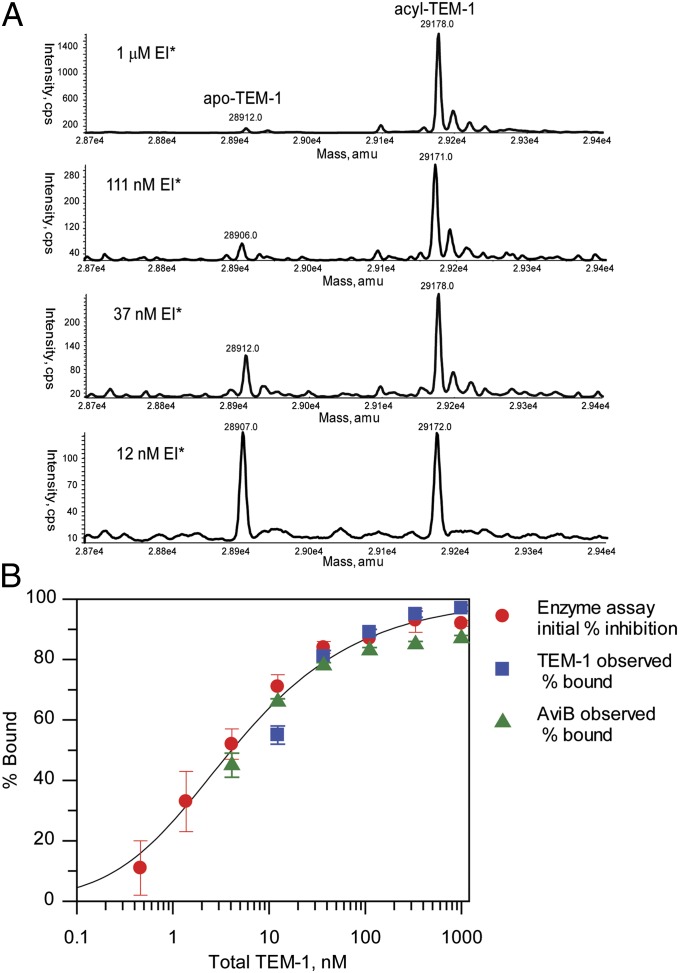
Fig. 5.
Equilibration of avibactam-TEM-1 acyl-enzyme. (A) Mass spectra of acylated TEM-1 (EI*) after dilution to various concentrations and equilibration for 2 h at 37 °C. (B) Fit of measurement of equilibria between avibactam-TEM-1 acyl-enzyme complex and free avibactam + TEM-1 as a function of complex dilution. The percent avibactam bound was measured by TEM-1 protein MS (blue squares), avibactam MS (green triangles), and initial enzyme activity (red circles). For the avibactam MS titration, the observed % free avibactam was used to calculate the % bound by assuming a mass balance that fraction bound is equal to (1 − fraction unbound). Error bars shown are ±SEM from three measurements for each detection technique. For calculation of Ki*, data for the different detection methods were fit independently assuming the tight-binding condition (39). The value of Ki* determined by the three techniques is 2.1 ± 1.0 nM (mean ± 2 SD), and the solid line indicates the fit to this value.