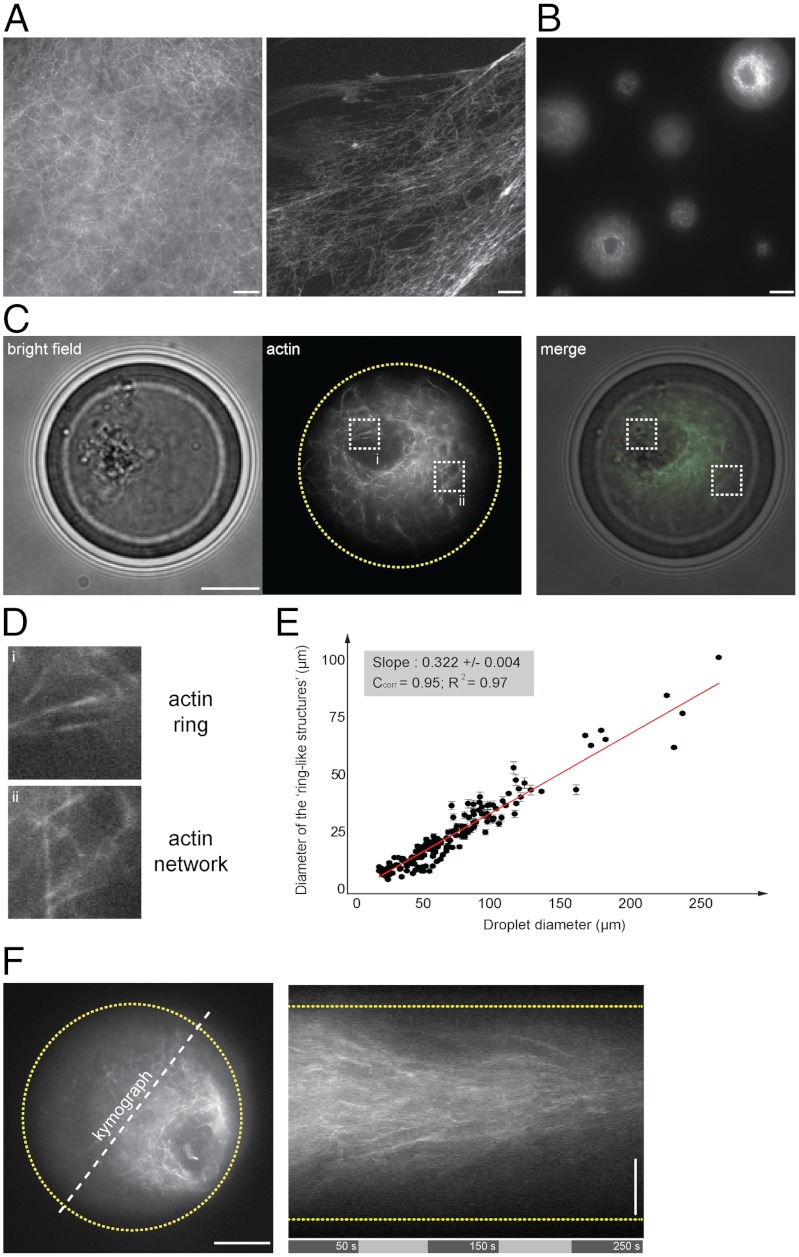
Fig. 1.
Symmetry breaking of confined F-actin network. (A) Fluorescent observation of F-actin network (in presence of Alexa488-conjugated phalloidin) generated in bulk extracts (5 min incubation). (B) Fluorescent observation of F-actin network within extract-in-oil droplets. (C, D) Bright field and fluorescent observations of F-actin network confined within a 34 μm-diameter droplet. (D) Actin filaments organized in a ring-like structure asymmetrically positioned within the droplet and surrounded by an actin cloud (actin network). (E) Plot of the ring-like structure diameter as a function of the droplet diameter shows a linear correlation between the ring and the droplet diameter. Quantifications of the ring diameters were performed by extracting a plot profile of the fluorescent structure. The droplet diameter was measured using bright field illumination. We estimated the precision of such measurements to 1 μm. (F) Representative kymograph illustrating the directional flow dynamics of F-actin network within a 40 μm-diameter droplet. Scale bars, 10 μm.