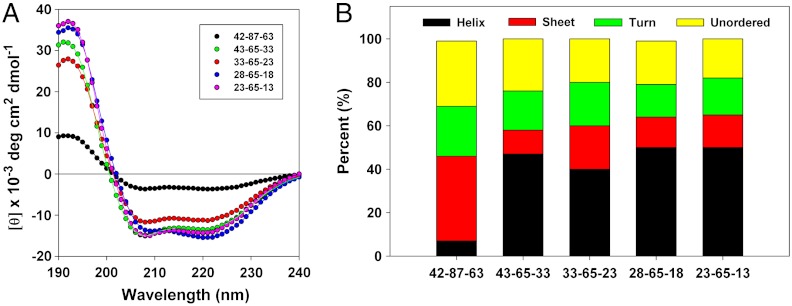
Fig. 4.
Far UV CD spectrometry of wild-type oleosin and the P = 65 family. (A) Far-UV circular dichroism spectra of wild-type oleosin and the P = 65 mutants. Solid lines represent fits calculated with DichroWeb software. The fits match experimental data very well allowing for prediction of overall secondary structure. (B) Estimation of secondary structure of wild-type oleosin and the P = 65 mutants. The P = 65 family show similar secondary structure, although mutants show increased alpha-helical structure compared to wild-type oleosin. Morphological differences in assemblies observed among different recombinant proteins are not attributable to changes in the secondary structure of the proteins across the P = 65 family.