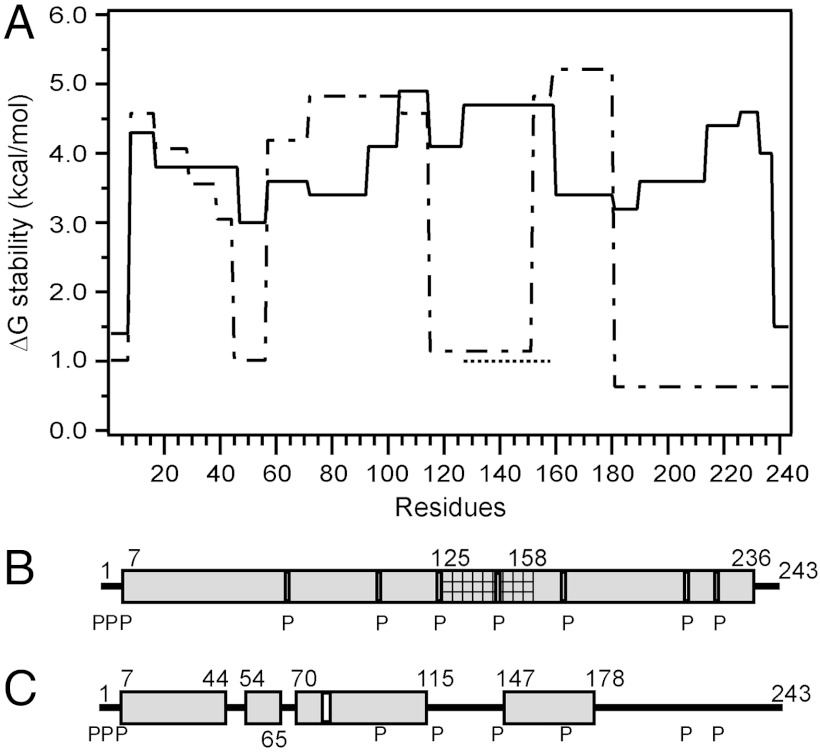
Fig. 2.
Summary of the HX-derived secondary structure assignments and α-helix stabilities for apoA-I in lipid-free and lipid-bound (9.6-nm discoidal HDL particle) states. (A) Site-resolved stability of lipid-free (dashed line) (from ref. 19) and lipid-bound (solid line) apoA-I. The HX kinetic data (pD 7.3, 5 °C) for the 9.6-nm discoidal HDL particle in Fig. S3 and Table S1 were analyzed to obtain Pf values for each apoA-I peptide. Residues 125–158 (cross-hatched in B) exhibit bimodal HX kinetics (see Fig. S8) indicating two populations undergoing HX at different rates; the ΔGstability for both states is shown (the dotted line at ΔG approximately 1 kcal/mol represents the fast state; see text). The disordered loop structure formed by the region around residues 125–158 is not included in B. B and C compare the HX-derived helix locations in lipid-bound (9.6-nm discoidal HDL particle) and lipid-free apoA-I, respectively. The gray cylinders represent α-helices, and the lines indicate disordered secondary structure. The positions of proline residues (P), whose presence leads to some perturbation of α-helix organization, are marked.