Table 1.
Optimization of the reaction conditions.
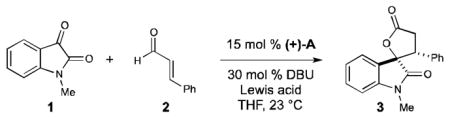
| ||||
|---|---|---|---|---|
| entry | variation of the standard conditions | conv[a] | dr[a] | ee[b] |
| 1 | no Lewis acid | 99 | 1.1:1 | 34 |
| 2 | Mg(OtBu)2 (50 mol %) | 99 | 1.5:1 | 35 |
| 3 | Ti(OiPr)4 (50 mol %) | 99 | 1:1 | 52 |
| 4 | LiCl (50 mol %) | 99 | 1.8:1 | 70 |
| 5 | LiCl (1 equiv) | 99 | 2:1 | 77 |
| 6 | LiCl (2 equiv) | 99 | 2.5:1 | 90 |
| 7 | LiCl (2 equiv), 12-crown-4 (4 equiv) | 99 | 1.2:1 | 35 |
| 8 | NaCl (2 equiv) | 99 | 1.2:1 | 53 |
| 9 | KCl (2 equiv) | 99 | 1:1 | 47 |
| 10 | LiBF4 (2 equiv) | 99 | 1:1 | 62 |
| 11 | LiOTf (2 equiv) | 99 | 3.7:1 | 39 |
| 12 | (+)-B, LiCl (2 equiv) | 99 | 2.8:1 | 92 |
| 13 | (−)-C, LiCl (2 equiv) | 66 | 1.1:1 | 38 |
| 14 | (−)-D, LiCl (2 equiv) | 99 | 1.7:1 | 32 |
| 15 | (−)-E, LiCl (2 equiv) | 99 | 1.4:1 | 50 |
| 16 | (+)-B (5 mol %), DBU (10 mol %) LiCl (2 equiv) | 99 | 2.4:1 | 96 |
|
| ||||
|
| ||||
Determined by 1H NMR spectroscopy (500 MHz) of the unpurified reaction.
Enantiomeric excess determined by HPLC.
