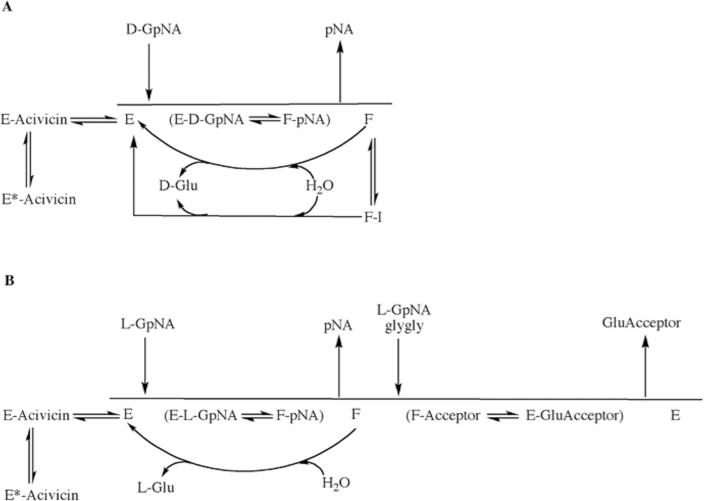
Fig. 8.
Proposed overall kinetic mechanism of inhibition and activation of GGT. Mechanism of hydrolysis of D-GpNA (A). Enzyme (E) is g-glutamylated by D-GpNA with release of pNA to give a form of the enzyme (F) that can transfer the g-glutamyl moiety to water. D-GpNA is not capable of acting as an acceptor. The ester bond can be hydrolyzed with rate constant khyd, and this rate is decreased in the presence of an inhibitor such as OU749 (F-I), or increased in the presence of an activator such as Compound 11 giving rate k'hyd. Acivicin can bind to the free enzyme at the acceptor site, and is slowly trapped (tightly bound) on enzyme. Mechanism of transpeptidation of GpNA (B). Enzyme (E) is g-glutamylated by GpNA with release of pNA to give a form of the enzyme (F) that can transfer the g-glutamyl moiety to an acceptor (L-GpNA and/or glygly). The ester bond can be hydrolyzed with rate constant khyd, but competition between water, L-GpNA and/or glygly decreases the rate of hydrolysis. As in panel A, acivicin can bind to the free enzyme at the acceptor site, and is slowly trapped on enzyme.