Table 3.
Kinetic Parameters for Activators of the D-GpNA Hydrolysis Reaction and Inhibition of the L-GpNA Transpeptidation Reaction.
| |||||||
|---|---|---|---|---|---|---|---|
| Hydrolysis D-GpNA | Inhibition L-GpNA | ||||||
| Compound # | Substitution | Voa (mM/min/nM) | V∞ (mM/min/nM) | KAct (μM) | Fold Activation | Transpeptidation L-GpNA Kii (μM) | Transpeptidation glygly Kis (μM) |
| 13 | None | 1.59 ± 0.06 | 9.09 ± 0.55 | 54.4 ± 3.0 | 5.72 ± 0.32 | 111.5 ± 5.5 | 59.6 ± 3.7 |
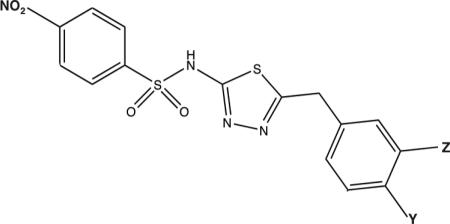
| 14 | Y = NO2 | 1.27 ± 0.01 | 5.08 ± 0.41 | 51.9 ± 0.8 | 4.00 ± 0.11 | 132.5 ± 5.5 | 81.2 ± 8.8 |
| 15 | Z = Cl | 1.30 ± 0.01 | 5.86 ± 0.23 | 38.6 ± 0.3 | 4.51 ± 0.05 | 119.1 ± 7.1 | 43.8 ± 7.7 |
| 11b | Y = OCH3 | 1.59 ± 0.14 | 8.63 ± 1.21 | 37.1 ± 5.6 | 5.43 ± 2.04 | 92.8 ± 4.3 | 95.7 ± 7.6 |
| 16 | Y = Cl | 1.71 ± 0.09 | 7.70 ± 0.46 | 31.4 ± 2.2 | 4.50 ± 0.63 | 61.9 ± 2.4 | 44.4 ± 3.4 |
| 17 | Y = CH3 | 2.62 ± 0.79 | 11.25 ± 4.27 | 25.3 ± 7.6 | 4.29 ± 2.20 | 112.5 ± 5.2 | 56.9 ± 8.3 |
| 18 | Z = CH3 | 1.15 ± 0.01 | 5.08 ± 0.20 | 25.2 ± 0.2 | 4.42 ± 0.64 | 121.5 ± 8.8 | 34.3 ± 0.9 |
| 19 | Y = F | 1.53 ± 0.09 | 5.39 ± 0.28 | 22.2 ± 2.0 | 3.52 ± 0.60 | Time Dependent | 31.8 ± 0.7 |
| 20c | Y = Cl Z = Cl |
-- | -- | -- | -- | Time Dependent | 10.3 ± 0.5 |
Vo = maximum velocity at zero activator; V∞ = Vo(KID/KIN) in eq. 2, maximum rate at infinite activator; Kact, activation constant or concentration that gives ½(V∞ + Vo), KID in eq. 2; fold activation = V∞/Vo.
Normalization of all values to Compound 11 yielded values similar to Compound 11
Compound 20 competitively inhibited the hydrolysis reaction with a Kii with D-GpNA of 78.2 ± 1.9 μM.
