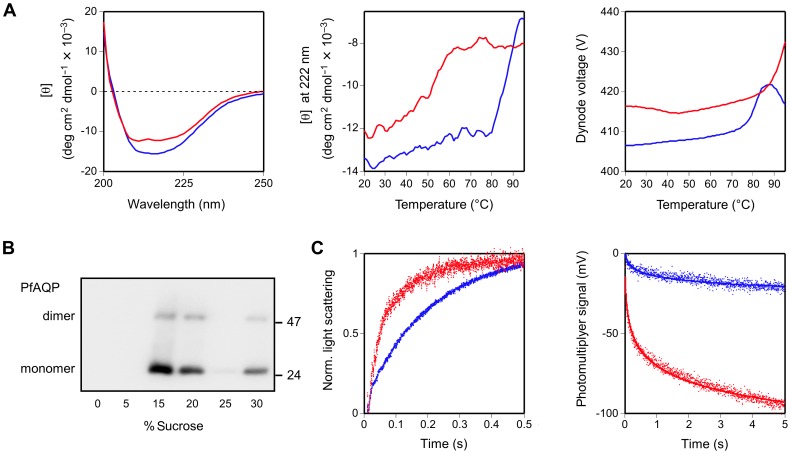
Figure 2. Structural and functional analysis of cell-free produced PfAQP without a GFP fusion.
A. PfAQP in the presence of Brij35 (blue curve) and Brij78 (red curve) was analyzed by circular dichroism. The left panel shows the mean residue molar ellipticity [θ] in the range of 200–250 nm. Thermal unfolding was monitored at 222 nm from 20°C to 95°C (middle panel). A plot of the photomultiplyer dynode voltage versus temperature (right panel) indicates an increase in turbidity at 80°C in the sample with Brij35 solubilized PfAQP suggesting protein agglomeration. B. Reconstitution of PfAQP, produced in the prescence of Brij78, into proteoliposomes was controlled by sucrose density gradient centrifugation and Western blot using an anti-His5 antiserum. The fractions with 15% and 20% sucrose contained reconstituted liposomal PfAQP; the 30% sucrose fraction displays precipitated, non-integrated PfAQP protein. PfAQP monomers (≈25 kDa) and dimers are visible. C. For functional analysis, PfAQP-proteoliposomes (red traces) and empty control liposomes (blue traces) were subjected to an outward osmotic gradient of 300 mosm kg−1 (left panel) and an inward isotonic glycerol gradient of 300 mM (right panel). Changes in the light scattering intensity reflect liposome shrinkage due to water efflux (increase in light scattering) and liposome swelling due to glycerol plus secondary water influx (decrease in light scattering). Note the difference of the scale of the abscissae as a consequence of lower glycerol permeability by at least one order of magnitude. The slow glycerol flux across the plain lipid liposome membrane did not reach a plateau, hence, the photomultiplyer signal was plotted without normalization. For each experiment nine traces were averaged and fitted to single exponential functions.