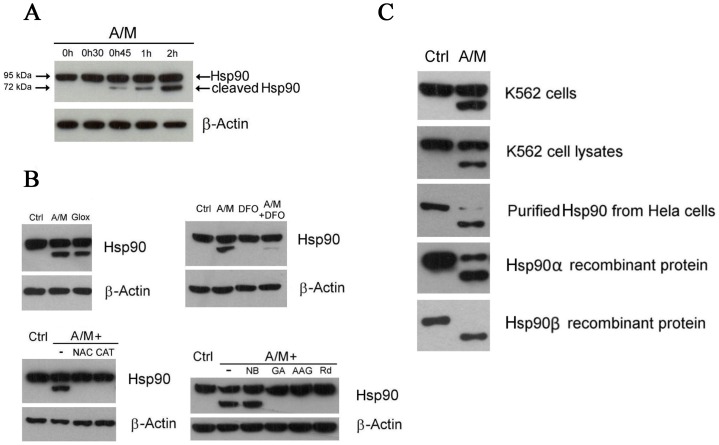
Figure 1. Oxidative stress leads to Hsp90 cleavage and client protein degradation.
(A) Time course of Hsp90 cleavage in K562 cells upon treatment with A/M (2 mM/10 µM). Hsp90 was detected with an anti-C terminus antibody from Santa Cruz Biotechnology (Hsp90 α/β, clone F-8). (B) Hsp90 cleavage in cells incubated for 2 hours with either A/M (2 mM/10 µM) or Glox (30 mM glucose/0.25 U/ml glucose oxidase), another H2O2-generating system (top left). Suppression of Hsp90 cleavage in cells preincubated for 1 h with 3 mM N-acetylcysteine (NAC) or 100 U/ml of catalase (CAT) and then exposed to A/M for 2 h (bottom left). Iron chelation by preincubating cells with deferoxamine (DFO) for 18 h decreased the cleavage induced by 2 h treatment with A/M (top right). Preincubation of cells for 1 h with 10 µM of N-term Hsp90 inhibitors, like geldanamycin (GA), 17-AAG (AAG) or radicicol (Rd), protected Hsp90 from the cleavage induced by 2 h treatment with A/M. Novobiocin (NB) at 1 mM did not protect (bottom right). Hsp90 was detected with the same antibody as in (A). (C) Cellular Hsp90 cleavage was reproduced in K562 cell lysates, in purified Hsp90 from HeLa cells, and in Hsp90 α and β recombinant proteins. K562 cells were incubated for two hours in the absence (Ctrl) or in the presence of A/M (2 mM/10 µM). K562 cell lysates (100 µg) were incubated for 1 h in the absence (Ctrl) or in the presence of A/M supplemented with ADP (0.2 mM) and FeCl3 (0.5 mM). For the experiments with purified and recombinant proteins, we used 2 µg of Hsp90, incubation lasted 30 min and A/M (2 mM/10 µM) was supplemented with 0.2 mM ADP and 0.5 mM FeCl3. Note that Hsp90 was detected with the same antibody as in (A) with the exception of recombinant Hsp90α that was detected with an anti-penta-His antibody.