Fig. 7.
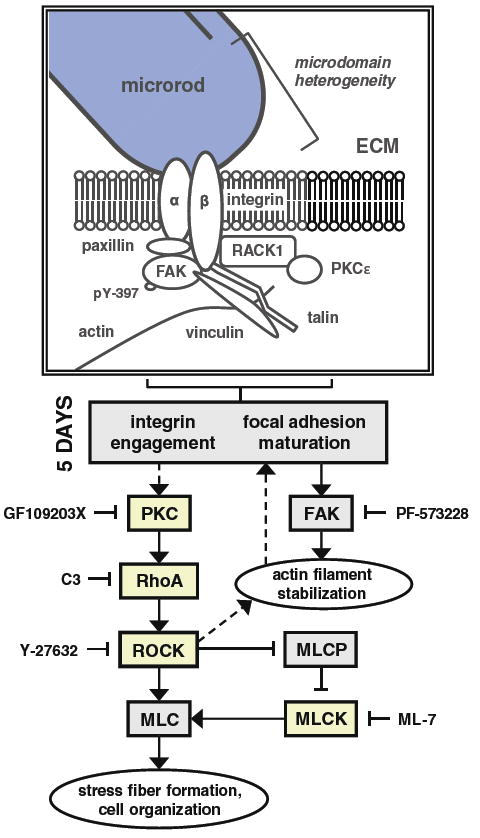
Possible mechanism of microrod-affected mechanics in cardiac myocytes. The schematic depicts the various signaling molecules that have demonstrated an involvement in the recognition of microrods, based on measurements of axial displacement in spontaneously beating myocytes. Microrods in 3D gels introduce local heterogeneity, which ultimately elicits differences in maximum displacement and shortening velocity by 5 days in culture. While focal adhesions are likely to mature soon after cell seeding, it is the maintenance of PKC and RhoA/ROCK signaling that appears to mediate the microrod-related changes in contractile behavior. As such, these changes were abrogated upon ≥24-h inhibition of RhoA, ROCK, MLCK, and PKC by C3 Transferase, Y-27632, ML-7, and GF109203X, respectively. However, targeting of active FAK kinase (identified by phosphorylation at tyrosine residue 397) from day 2 to day 5 with PF-573228 did not prevent the response to microrods. This suggests that the required activity of RhoA/ROCK is downstream of PKC but not FAK. Yet curiously, PKC is known to be regulated both upstream and downstream of FAK signaling (Ruwhof et al. 2001; Heidkamp et al. 2003). The major calcium-independent isoform of PKC in the heart, PKCε, has been shown to bind receptor for activated C-kinase-1 (RACK1), which concurrently associates with β-integrin subunits; the complex promotes integrin clustering and focal adhesion development (Mochly-Rosen et al. 1991; Besson et al. 2002). In the diagram, proteins in boxes shaded yellow had activities that were found to participate in the effects of microrods
