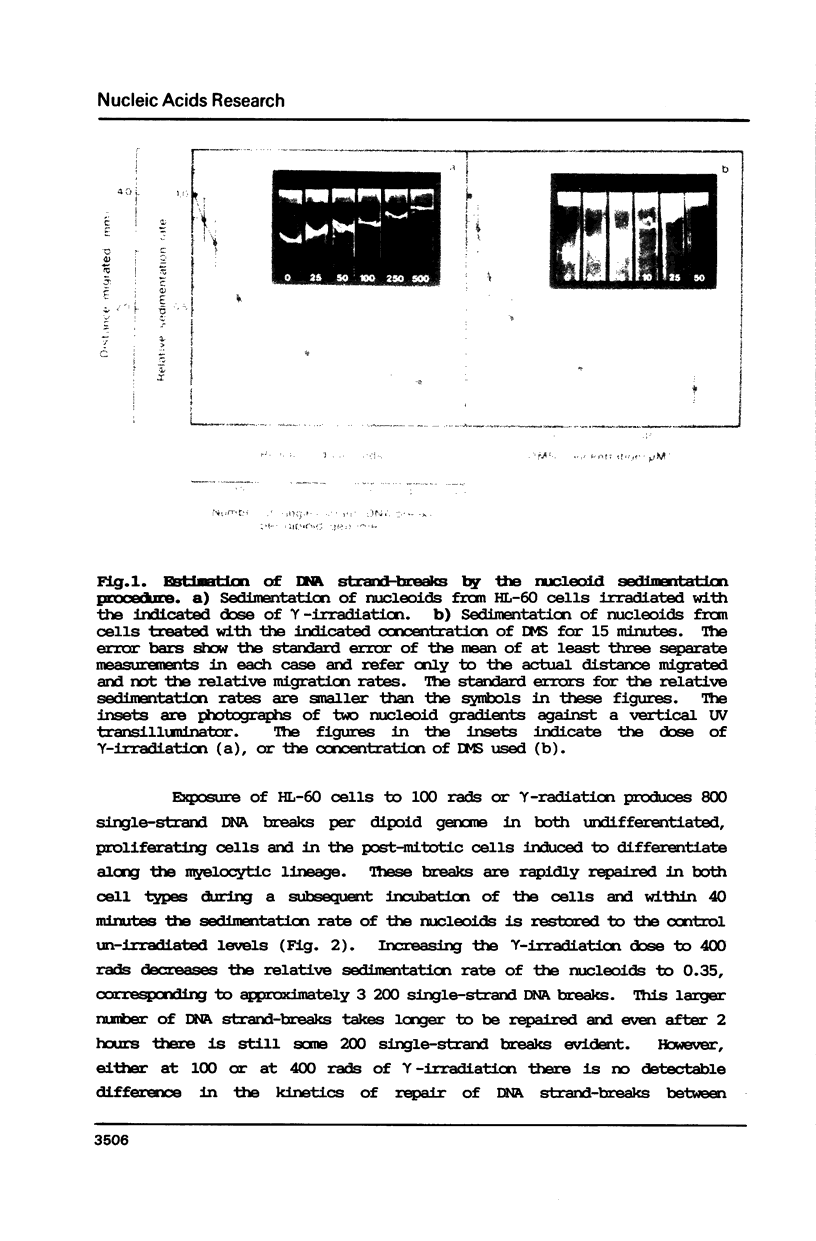
Abstract
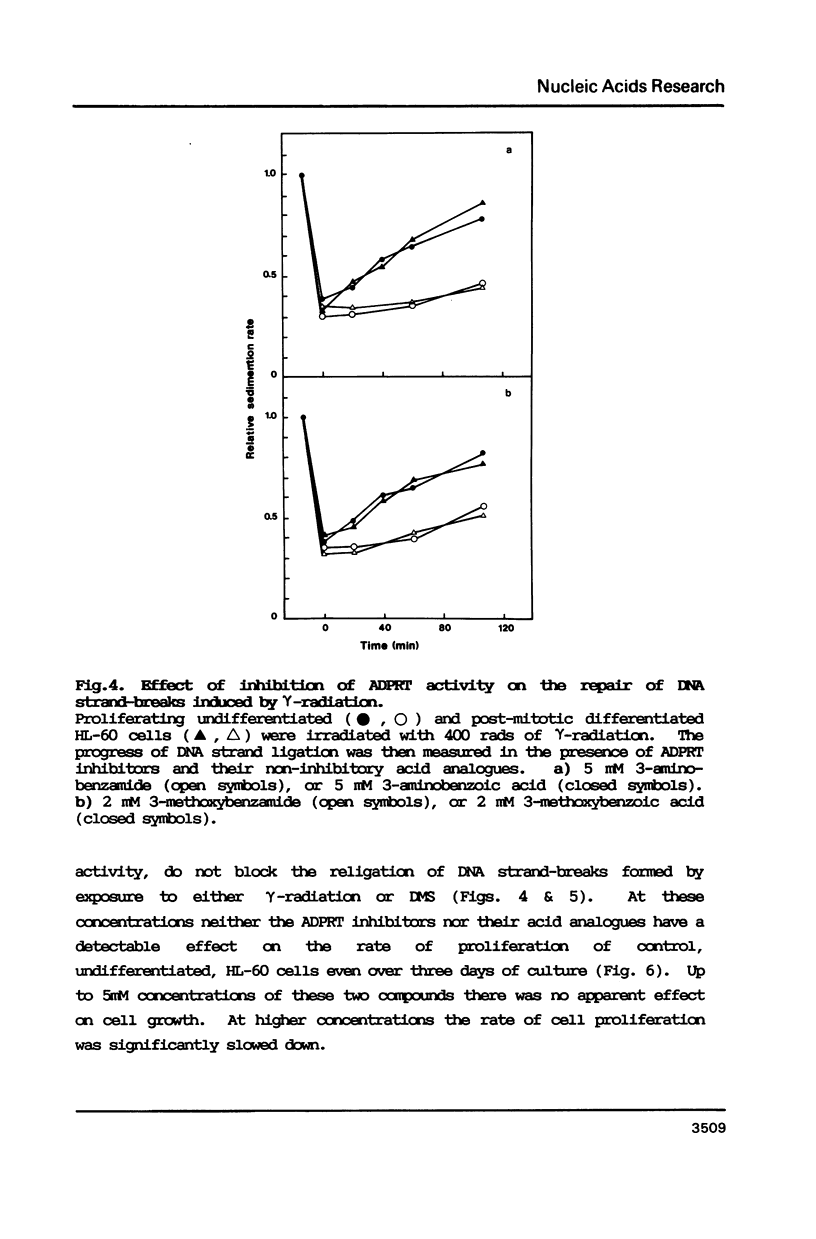
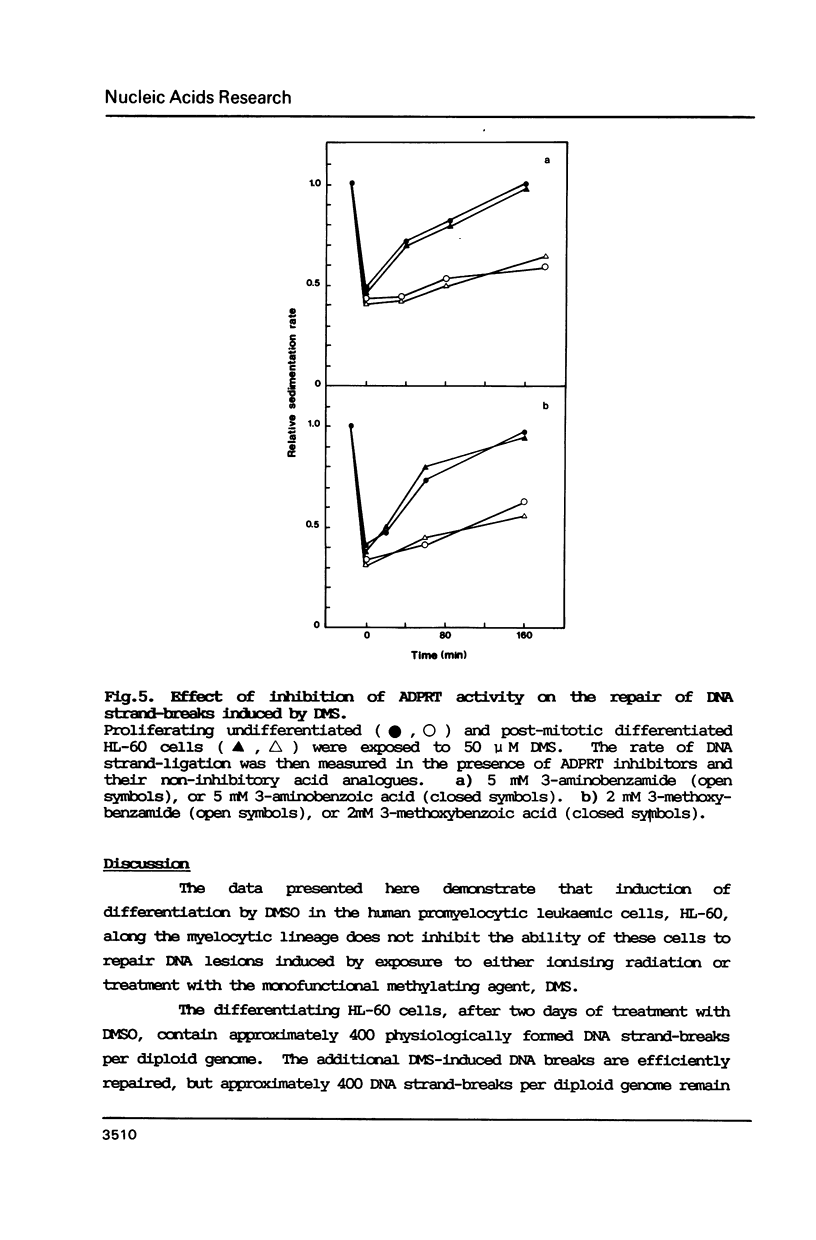
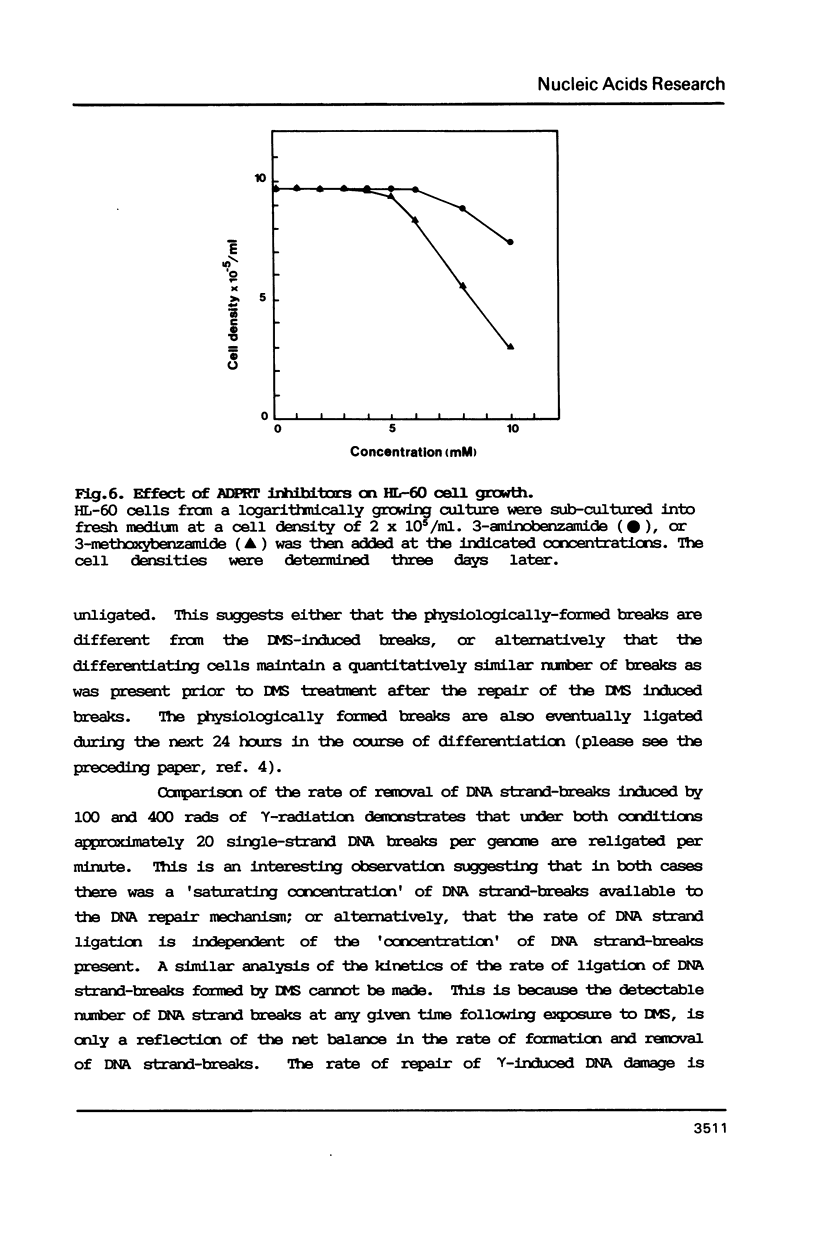
The human promyelocytic cell line, HL-60, shows large changes in endogenous poly(ADP-ribose) and in nuclear ADP-ribosyl transferase activity (ADPRT) during its induced myelocytic differentiation. DNA strand-breaks are an essential activator for this enzyme; and transient DNA strand breaks occur during the myelocytic differentiation of HL-60 cells. We have tested the hypothesis that these post-mitotic, terminally differentiating cells are less efficient in DNA repair, and specifically in DNA strand rejoining, than their proliferating precursor cells. We have found that this hypothesis is not tenable. We observe that there is no detectable reduction in the efficiency of DNA excision repair after exposure to either dimethyl sulphate or gamma-irradiation in HL-60 cells induced to differentiate by dimethyl sulphoxide. Moreover, the efficient excision repair of either dimethyl sulphate or gamma-irradiation induced lesions, both in the differentiated and undifferentiated HL-60 cells, is blocked by the inhibition of ADPRT activity.
Full text
PDF


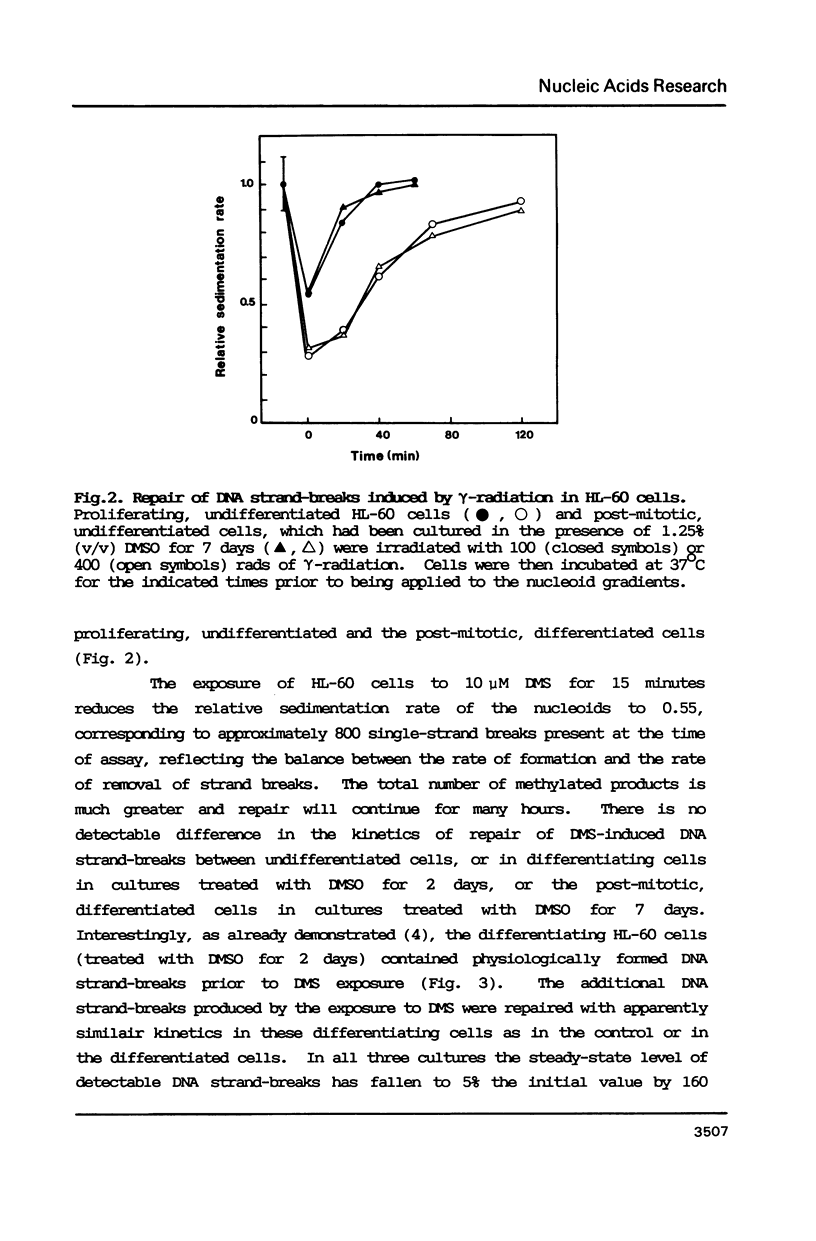


Images in this article
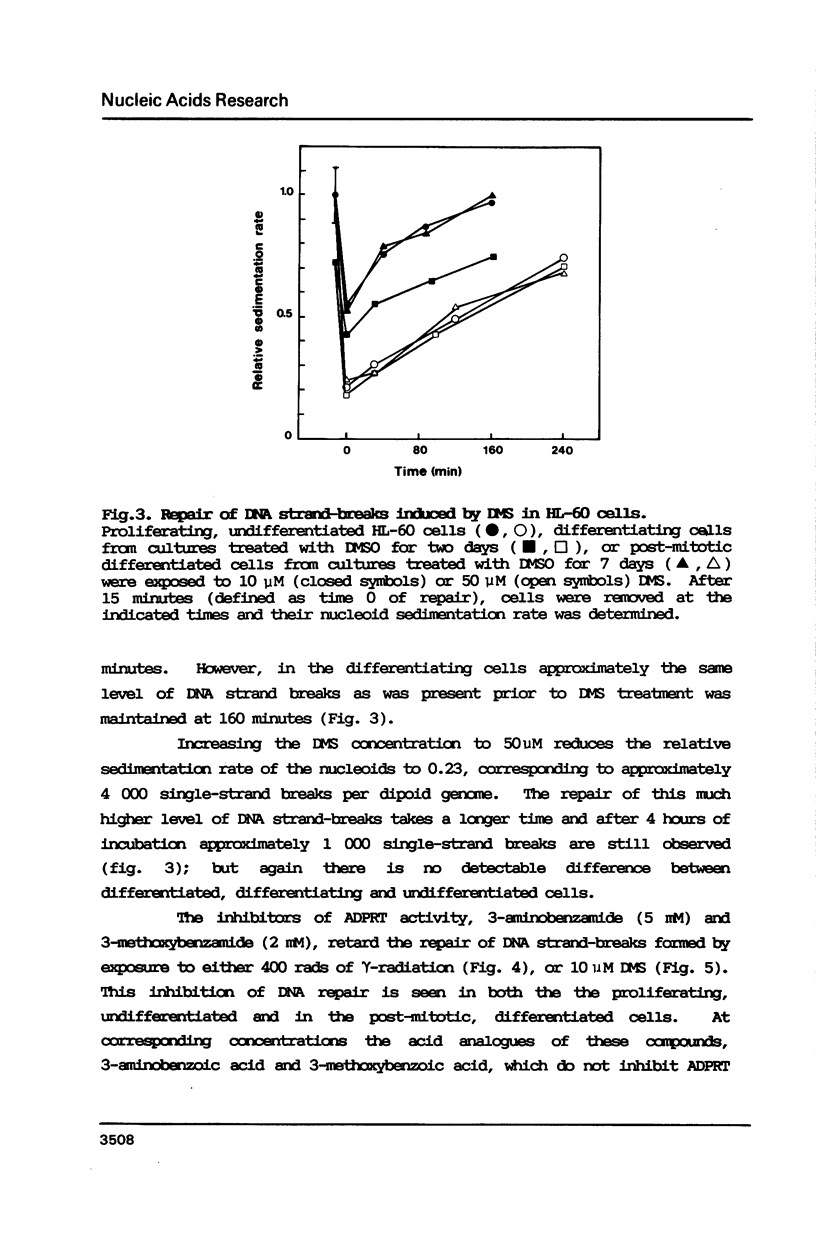
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Borek C., Morgan W. F., Ong A., Cleaver J. E. Inhibition of malignant transformation in vitro by inhibitors of poly(ADP-ribose) synthesis. Proc Natl Acad Sci U S A. 1984 Jan;81(1):243–247. doi: 10.1073/pnas.81.1.243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byfield J. E., Lee Y. C., Klisak I., Finklestein J. Z. Effect of differentiation on the repair of DNA single strand breaks in neuroblastoma cells. Biochem Biophys Res Commun. 1975 Apr 7;63(3):730–735. doi: 10.1016/s0006-291x(75)80444-x. [DOI] [PubMed] [Google Scholar]
- Chan A. C., Walker I. G. Reduced DNA repair during differentiation of a myogenic cell line. J Cell Biol. 1976 Sep;70(3):685–691. doi: 10.1083/jcb.70.3.685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epstein J. H., Fukuyama K., Epstein W. L. UVL induced stimulation of DNA synthesis in hairless mouse epidermis. J Invest Dermatol. 1968 Dec;51(6):445–453. doi: 10.1038/jid.1968.154. [DOI] [PubMed] [Google Scholar]
- Farzaneh F., Meldrum R., Shall S. Transient formation of DNA strand breaks during the induced differentiation of a human promyelocytic leukaemic cell line, HL-60. Nucleic Acids Res. 1987 Apr 24;15(8):3493–3502. doi: 10.1093/nar/15.8.3493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farzaneh F., Zalin R., Brill D., Shall S. DNA strand breaks and ADP-ribosyl transferase activation during cell differentiation. Nature. 1982 Nov 25;300(5890):362–366. doi: 10.1038/300362a0. [DOI] [PubMed] [Google Scholar]
- Francis G. E., Gray D. A., Berney J. J., Wing M. A., Guimaraes J. E., Hoffbrand A. V. Role of ADP-ribosyl transferase in differentiation of human granulocyte-macrophage progenitors to the macrophage lineage. Blood. 1983 Nov;62(5):1055–1062. [PubMed] [Google Scholar]
- Hahn G. M., King D., Yang S. J. Quantitative changes in unscheduled DNA synthesis in rat muscle cells after differentiation. Nat New Biol. 1971 Apr 21;230(16):242–244. doi: 10.1038/newbio230242a0. [DOI] [PubMed] [Google Scholar]
- Johnstone A. P., Williams G. T. Role of DNA breaks and ADP-ribosyl transferase activity in eukaryotic differentiation demonstrated in human lymphocytes. Nature. 1982 Nov 25;300(5890):368–370. doi: 10.1038/300368a0. [DOI] [PubMed] [Google Scholar]
- Kanai M., Miwa M., Kondo T., Tanaka Y., Nakayasu M., Sugimura T. Involvement of poly (ADP-ribose) metabolism in induction of differentiation of HL-60 promyelocytic leukemia cells. Biochem Biophys Res Commun. 1982 Mar 30;105(2):404–411. doi: 10.1016/0006-291x(82)91448-6. [DOI] [PubMed] [Google Scholar]
- Karran P., Moscona A., Strauss B. Developmental decline in DNA repair in neural retina cells of chick embryos. Persistent deficiency of repair competence in a cell line derived from late embryos. J Cell Biol. 1977 Jul;74(1):274–286. doi: 10.1083/jcb.74.1.274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karran P., Ormerod M. G. Is the ability to repair damage to DNA related to the proliferative capacity of a cell? The rejoining of X-ray-produced strand breaks. Biochim Biophys Acta. 1973 Feb 23;299(1):54–64. doi: 10.1016/0005-2787(73)90397-3. [DOI] [PubMed] [Google Scholar]
- Kaufman S. J., Teplinsky K. S., Koval T. M. A re-evaluation of DNA repair during skeletal myogenesis in vitro. Exp Cell Res. 1980 Nov;130(1):41–48. doi: 10.1016/0014-4827(80)90040-3. [DOI] [PubMed] [Google Scholar]
- Mattern M. R., Cerutti P. A. Age-dependent excision repair of damaged thymine from gamma-irradiated DNA by isolated nuclei from human fibroblasts. Nature. 1975 Apr 3;254(5499):450–452. doi: 10.1038/254450a0. [DOI] [PubMed] [Google Scholar]
- Moreno G., Vinzens F., Prunieras M. UV-induced unscheduled DNA synthesis in guinea pig skin melanocytes isolated in culture. J Invest Dermatol. 1978 Jan;70(1):21–24. doi: 10.1111/1523-1747.ep12543362. [DOI] [PubMed] [Google Scholar]
- Ormerod M. G., Stevens U. The rejoining of x-ray-induced strand breaks in the DNA of a murine lymphoma cell (L5178Y). Biochim Biophys Acta. 1971 Feb 25;232(1):72–82. doi: 10.1016/0005-2787(71)90492-8. [DOI] [PubMed] [Google Scholar]
- Purnell M. R., Whish W. J. Novel inhibitors of poly(ADP-ribose) synthetase. Biochem J. 1980 Mar 1;185(3):775–777. doi: 10.1042/bj1850775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scher W., Friend C. Breakage of DNA and alterations in folded genomes by inducers of differentiation in Friend erythroleukemic cells. Cancer Res. 1978 Mar;38(3):841–849. [PubMed] [Google Scholar]
- Smith C. A., Hanawalt P. C. Repair replication in cultured normal and transformed human fibroblasts. Biochim Biophys Acta. 1976 Oct 4;447(2):121–132. doi: 10.1016/0005-2787(76)90335-x. [DOI] [PubMed] [Google Scholar]
- Stockdale F. E. DNA synthesis in differentiating skeletal muscle cells: initiation by ultraviolet light. Science. 1971 Mar 19;171(3976):1145–1147. doi: 10.1126/science.171.3976.1145. [DOI] [PubMed] [Google Scholar]
- Terada M., Nudel U., Fibach E., Rifkind R. A., Marks P. A. Changes in DNA associated with induction of erythroid differentiation by dimethyl sulfoxide in murine erythroleukemia cells. Cancer Res. 1978 Mar;38(3):835–840. [PubMed] [Google Scholar]
- Wheeler K. T., Lett J. T. On the possibility that DNA repair is related to age in non-dividing cells. Proc Natl Acad Sci U S A. 1974 May;71(5):1862–1865. doi: 10.1073/pnas.71.5.1862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wheeler K. T., Sheridan R. E., Pautler E. L., Lett J. T. In vivo restitution of the DNA structure in gamma irradiated rabbit retinas. Radiat Res. 1973 Mar;53(3):414–427. [PubMed] [Google Scholar]
- Williams G. T., Johnstone A. P. ADP-ribosyl transferase, rearrangement of DNA, and cell differentiation. Biosci Rep. 1983 Sep;3(9):815–830. doi: 10.1007/BF01133780. [DOI] [PubMed] [Google Scholar]