Abstract
A novel 15C compound, pseudoalteromone B (1), possessing a novel carbon skeleton, was obtained from a marine bacterium Pseudoalteromonas sp. CGH2XX. This bacterium was originally isolated from a cultured-type octocoral Lobophytum crassum, that was growing in cultivating tanks equipped with a flow-through sea water system. The structure of 1 was established by spectroscopic methods. Pseudoalteromone B (1) displayed a modestly inhibitory effect on the release of elastase by human neutrophils.
Keywords: pseudoalteromone, Pseudoalteromonas, anti-inflammatory, Lobophytum crassum, elastase
1. Introduction
Marine bacteria belonging to the genus Pseudoalteromonas sp. (family Pseudoalteromonadaceae) have proven to be not only an important source of various antibiotics, but have also played an interesting role in marine ecology [1,2,3,4]. In the continuing research aimed at the discovery of new natural substances from marine microorganisms, an organic extract of the bacterium identified as Pseudoalteromonas sp. CGH2XX, which was originally isolated from a cultured-type octocoral Lobophytum crassum (family Alcyonacea), exhibited significant cytotoxicity toward the HL-60 (human acute promyelocytic leukemia) and CCRF-CEM (human T cell acute lymphoblastic leukemia) tumor cells (IC50 = 0.9, 1.2 µg/mL) and displayed a significant inhibitory effect (inhibition rate 45.1%) on the release of elastase by human neutrophils at a concentration of 10 µg/mL. We isolated a novel 15C compound, pseudoalteromone B (1) (Figure 1), from this microorganism. The structure of 1 was established by spectroscopic methods and this compound displayed a modestly inhibitory effect on the release of elastase by human neutrophils.
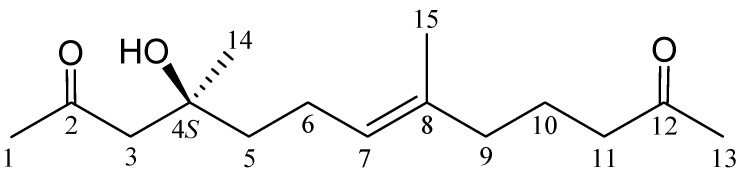
Figure 1.
The structure of pseudoalteromone B (1).
2. Results and Discussion
Pseudoalteromone B (1) was isolated as an oil and had the molecular formula C15H26O3, as determined by HRESIMS (C15H26O3 + Na, m/z found 277.1779, calculated 277.1780) indicating three degrees of unsaturation. The IR absorption bands at 3502 and 1706 cm−1 were characteristic for the hydroxy and ketone groups.
Table 1.
1H (400 MHz, CDCl3) and 13C (100 MHz, CDCl3) NMR data for 1.
| Position | δH (J in Hz) | δC, Mult. |
|---|---|---|
| 1 | 2.18 s | 31.9, CH3 |
| 2 | 211.0, qC | |
| 3a/b | 2.58 d (17.2); 2.65 d (17.2) | 52.3, CH2 |
| 4 | 71.5, qC | |
| 5 | 1.51 m | 41.9, CH2 |
| 6 | 2.04 m | 22.5, CH2 |
| 7 | 5.09 tq (7.2, 1.2) | 124.8, CH |
| 8 | 134.6, qC | |
| 9 | 1.96 t (7.2) | 38.8, CH2 |
| 10 | 1.66 quintet (7.2) | 21.8, CH2 |
| 11 | 2.37 t (7.2) | 43.0, CH2 |
| 12 | 209.1, qC | |
| 13 | 2.12 s | 29.9, CH3 |
| 14 | 1.22 s | 26.7, CH3 |
| 15 | 1.58 br s | 15.7, CH3 |
The 1H and 13C NMR data of 1 (Table 1) showed the presence of 15 carbon signals, which were identified by the assistance of a DEPT spectrum as four methyls, six sp3 methylenes, an sp2 methine, an sp3 quaternary carbon, and three sp2 quaternary carbons including two ketone carbonyls. The 1H NMR spectrum of 1 showed a signal of olefinic proton (δH 5.09, 1H, tq, J = 7.2, 1.2 Hz, H-7), two acetyl methyls (δH 2.18, 3H, s, H3-1; 2.12, 3H, s, H3-13), a vinyl methyl (δH 1.58, 3H, br s, H3-15), a tertiary methyl attaching at an oxygenated quaternary carbon (δH 1.22, 3H, s, H3-14) and six pairs of methylene protons (δH 2.65, 1H, d, J = 17.2 Hz; 2.58, 1H, d, J = 17.2 Hz, H2-3; 2.37, 2H, t, J = 7.2 Hz, H2-11; 2.04, 2H, m, H2-6; 1.96, 2H, t, J = 7.2 Hz, H2-9; 1.66, 2H, quintet, J = 7.2 Hz, H2-10; 1.51, 2H, m, H2-5).
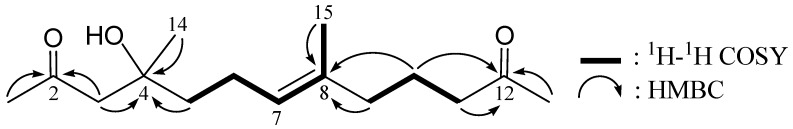
The constitution of the carbon skeleton of 1 was elucidated initially by the 1H–1H COSY and HMBC correlations of 1 (Figure 2), it was possible to establish the separate spin systems that map out the proton sequences from H2-5/H2-6/H-7 and H2-9/H2-10/H2-11. These data, together with the HMBC correlations between H3-1/C-2, C-3; H2-3/C-2, C-4, C-5; H2-5/C-4, C-6; H-7/C-9; H2-9/C-7, C-8, C-10, C-11; H2-10/C-8, C-9, C-11, C-12; H2-11/C-9, C-10, C-12; and H3-13/C-11, C-12, permitted elucidation of the main straight carbon skeleton. The vinyl methyl at C-8 was confirmed by the HMBC correlations between H-7, H2-9/C-15; and H3-15/C-7, C-8, C-9; and further supported by an allylic coupling between H-7 and H3-15 (J = 1.2 Hz). Based on these data, together with the HMBC correlations between H3-14/C-3, C-4, C-5 and H2-3, H2-5/C-14, the planar structure of 1 was established.
Figure 2.
The 1H–1H COSY and selective HMBC correlations (protons→quaternary carbons) of 1.
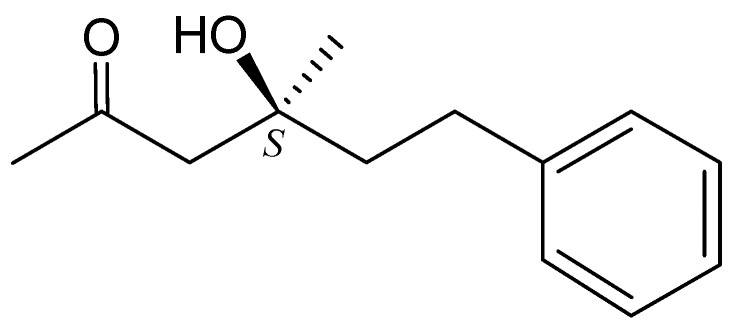
In the NOESY experiment of 1, a correlation between H-7 with H2-9, as well as the lack of correlation between H-7 and H3-15, reflected the E-configuration of C-7/8 double bond. Furthermore, by comparison of the rotation value of 1 ([α]23D −20 (c 0.03, CHCl3)) with that of a known synthetic compound, (S)-4-hydroxy-4-methyl-6-phenylhexan-2-one (2) ([α]25D −14.5 (c 1.1, CHCl3)) (Figure 3) [5], the absolute configuration for the C-4 chiral center of 1 was determined as S form as that of 2. Based on the above findings, the structure of 1 was determined unambiguously.
Figure 3.
The structure of (S)-4-hydroxy-4-methyl-6-phenylhexan-2-one (2).
The in vitro cytotoxicity of pseudoalteromone B (1) toward HCT116 (human colorectal carcinoma), K-562 (human chronic myelogenous leukemia), HL-60 (human acute promyelocytic leukemia), CCRF-CEM (human T cell acute lymphoblastic leukemia), T-47D (human breast ductal carcinoma), and MDA-MB-231 (human breast adenocarcinoma) cells was tested. Unfortunately, the new compound 1 described herein is not active toward the above cells (all IC50 values > 20 µg/mL). The in vitro anti-inflammatory effect of 1 was tested. Pseudoalteromone B (1) displayed a modestly inhibitory effect (inhibition rate 20.7%) on the release of elastase by human neutrophils at a concentration of 10 µg/mL.
3. Experimental Section
3.1. General Experimental Procedures
Optical rotations were measured on a Jasco P-1020 polarimeter. IR spectra were recorded on a Jasco FT/IR-4100 infrared spectrophotometer. The NMR spectra were recorded on a Varian Mercury Plus 400 FT-NMR at 400 MHz for 1H and 100 MHz for 13C, in CDCl3, respectively. Proton chemical shifts were referenced to the residual CHCl3 signal (δH 7.26 ppm). 13C NMR spectra were referenced to the center peak of CDCl3 at δC 77.1 ppm. ESIMS and HRESIMS data were recorded on a Bruker APEX II mass spectrometer. Silica gel (Merck, 230–400 mesh) and Sephadex LH-20 (Amersham Biosciences) were used for column chromatography. TLC was carried out on precoated Kieselgel 60 F254 (0.25 mm, Merck); spots were visualized by spraying with 10% H2SO4 solution followed by heating.
3.2. Marine Bacteria Isolation, Culture Conditions and Extract Preparation
A marine bacterium number CGH2XX was isolated from soft coral Lobophytum crassum that was growing in cultivating tanks equipped with a flow-through sea water system [4]. The bacterium strain CGH2XX was 98.3% identical with Pseudoalteromonas sp. H02P24-23 (Genebank accession no. HQ161380) on the basis of 16S rDNA gene sequence. The marine bacterium was cultured in 2.5 L flasks containing 1 L M1 broth (not containing agar) with 80% seawater. Flasks were incubated at 25 °C on a rotatory shaker at 120 rpm. After five days of incubation, extraction of the culture broth (10.0 L) with ethyl acetate (EtOAc, 2 × 10.0 L) yielded 1.71 g of crude extract. The extracts obtained were stored at −20 °C.
3.3. Separation
Crude extract was separated on Sephadex LH-20 and eluted using a mixture of dichloromethane and methanol (1:1) to yield 17 fractions. Fraction 6 was selected for further study and purified by silica gel, using a mixture of n-hexane and EtOAc (2:1) as a mobile phase to afford compound 1 (4.2 mg).
Pseudoalteromone B (1): colorless oil; [α]23D −20 (c 0.03, CHCl3); IR (neat) νmax 3502, 1706 cm−1; 1H (CDCl3, 400 MHz) and 13C (CDCl3, 100 MHz) NMR data, see Table 1; ESIMS: m/z 277 (M + Na)+; HRESIMS: m/z 277.1779 (calcd for C15H26O3 + Na, 277.1780).
3.4. Cytotoxicity Testing
The cytotoxicity of compound 1 was assayed with a modification of the MTT [3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide] colorimetric method. Cytotoxicity assays were carried out according to previously described procedures [6,7,8].
3.5. Elastase Release by Human Neutrophils
Human neutrophils were obtained by means of dextran sedimentation and Ficoll centrifugation. Elastase release experiments were performed using MeO-Suc-Ala-Ala-Pro-Valp-nitroanilide as the elastase substrate [9,10,11].
4. Conclusions
In a previous study [4], an ubiquinone derivative, pseudoalteromone A, was isolated from Pseudoalteromonas sp. CGH2XX, and this compound was found to be cytotoxic toward MOLT-4 (human acute lymphoblastic leukemia) and T-47D (human breast ductal carcinoma) cells (IC50 = 3.8, 4.0 µg/mL) and displayed moderately inhibitory effects on the generation of superoxide anion and the release of elastase (inhibition rates 38.0, 20.2%) by human neutrophils at a concentration of 10 µg/mL [12]. However, as described in the beginning of this communication, the organic extract of Pseudoalteromonas sp. CGH2XX showed significant cytotoxicity and anti-inflammatory activity. At this stage, the results showed that pseudoalteromone B (1) displayed a modestly anti-inflammatory activity and this compound was not cytotoxic toward HCT116, K-562, HL-60, CCRF-CEM, T-47D and MDA-MB-231 cells. We suggested that the other active components exist in the other fractions. The possible activity for pseudoalteromone B (1) will be studied if we can get enough material from Pseudoalteromonas sp. CGH2XX. Furthermore, to the best of our knowledge, compounds pseudoalteromones A and B, were the first two compounds from the marine bacterium belonging to the genus Pseudoalteromonas associated with octocorals.
Acknowledgments
This work was supported by grants from the National Dong Hwa University; the National Museum of Marine Biology and Aquarium (Grant No. 10120022); the Division of Marine Biotechnology, Asia-Pacific Ocean Research Center, National Sun Yat-sen University (Grant No. 00C-0302-05); and the National Science Council (Grant No. NSC 101-2325-B-291-001, 101-2320-B-291-001-MY3 and 98-2320-B-291-001-MY3), Taiwan, awarded to P.-J.S.
Footnotes
Samples Availability: Not available.
References and Notes
- 1.Bowman J.P. Bioactive compound synthetic capacity and ecological significance of marine bacterial genus Pseudoalteromonas. Mar. Drugs. 2007;5:220–241. doi: 10.3390/md504220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fehér D., Barlow R.S., Lorenzo P.S., Hemscheidt T.K. A 2-substituted prodiginine, 2-(p-hydroxybenzyl)prodigiosin, from Pseudoalteromonas rubra. J. Nat. Prod. 2008;71:1970–1972. doi: 10.1021/np800493p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fehér D., Barlow R., McAtee J., Hemscheidt T.K. Highly brominated antimicrobial metabolites from a marine Pseudoalteromonas sp. J. Nat. Prod. 2010;73:1963–1966. doi: 10.1021/np100506z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chen Y.-H., Lu M.-C., Chang Y.-C., Hwang T.-L., Wang W.-H., Weng C.-F., Kuo J., Sung P.-J. Pseudoalteromone A: a novel bioactive ubiquinone from a marine bacterium Pseudoalteromonas sp. CGH2XX (Pseudoalteromonadaceae) Tetrahedron Lett. 2012;53:1675–1677. [Google Scholar]
- 5.Chen I.-H., Kanai M., Shibasaki M. Copper(I)—Secondary diamine complex-catalyzed enantioselective conjugate boration of linear β,β-disubstituted enones. Org. Lett. 2010;12:4098–4101. doi: 10.1021/ol101691p. [DOI] [PubMed] [Google Scholar]
- 6.Doxorubicin was used as a reference compound in cytototxicity testing. Doxorubicin showed cytotoxicity toward HCT116, K-562, HL-60, CCRF-CEM, T-47D, and MDA-MB-231 cells (IC50 = 1.8, 0.8, 0.2, 0.1, 1.5 and 1.7 µg/mL).
- 7.Alley M.C., Scudiero D.A., Monks A., Hursey M.L., Czerwinski M.J., Fine D.L., Abbott B.J., Mayo J.G., Shoemaker R.H., Boyd M.R. Feasibility of drug screening with panels of human tumor cell lines using a microculture tetrazolium assay. Cancer Res. 1988;48:589–601. [PubMed] [Google Scholar]
- 8.Scudiero D.A., Shoemaker R.H., Paull K.D., Monks A., Tierney S., Nofziger T.H., Currens M.J., Seniff D., Boyd M.R. Evaluation of a soluble tetrazolium/formazan assay for cell growth and drug sensitivity in culture using human and other tumor cell lines. Cancer Res. 1988;48:4827–4833. [PubMed] [Google Scholar]
- 9.In the in vitro anti-inflammatory bioassay, the inhibitory effect on the release of elastase by activated neutrophils was used as an indicator. For significant activity of pure compounds, an inhibition rate ≥ 40% is required (inhibition rate ≤ 10%, not active; 20% ≥ inhibition rate ≥ 10%, weakly anti-inflammatory; 40% ≥ inhibition rate ≥ 20%, modestly anti-inflammatory). Elastatinal was used as a reference compound in anti-inflammatory activity test (IC50 = 31.9 µg/mL).
- 10.Hwang T.-L., Wang C.-C., Kuo Y.-H., Huang H.-C., Wu Y.-C., Kuo L.-M., Wu Y.-H. The hederagenin saponin SMG-1 is a natural FMLP receptor inhibitor that suppresses human neutrophil activation. Biochem. Pharmacol. 2010;80:1190–1200. doi: 10.1016/j.bcp.2010.06.028. [DOI] [PubMed] [Google Scholar]
- 11.Yu H.-P., Hsieh P.-W., Chang Y.-J., Chung P.-J., Kuo L.-M., Hwang T.-L. 2-(2-Fluorobenz-amido)benzoate ethyl ester (EFB-1) inhibits superoxide production by human neutrophils and attenuates hemorrhagic shock-induced organ dysfunction in rats. Free Radic. Biol. Med. 2011;50:1737–1748. doi: 10.1016/j.freeradbiomed.2011.03.026. [DOI] [PubMed] [Google Scholar]
- 12.The authors regret that there is an error in pages 1 and 2 of [4] (pages 1675 and 1676 of the issue). In [4], the ubiquinone, pseudoalteromone A, was reported to display an inhibitory effect on the release of elastase (inhibition rate 45.1%) by human nuetrophils at a concentration of 10 µg/mL. However, after detailed collating, we found this data was cited incorrectly. The data (inhibition rate 45.1%) expressed an inhibitory effect of an organic extract from the marine bacterium Pseudoalteromonas sp. CGH2XX on the release of elastase by human nuetrophils as presented in this study. The in vitro anti-inflammatory effects of pseudoalteromone A were tested again. Pseudoalteromone A displayed moderately inhibitory effects on the generation of superoxide anion and the release of elastase (inhibition rates 38.0% and 20.2%) by human neutrophils at a concentration of 10 µg/mL. Diphenyl indonium (DPI) and elastatinal were used as reference compounds in anti-inflammatory activity testing. DPI displayed an inhibitory effect on superoxide anion generation (IC50 = 0.9 µg/mL), and elastatinal exhibited an inhibitory effect on elastase release (IC50 = 31.9 µg/mL) by human neutrophils, respectively. The authors apologize for any inconvenience caused by this error.