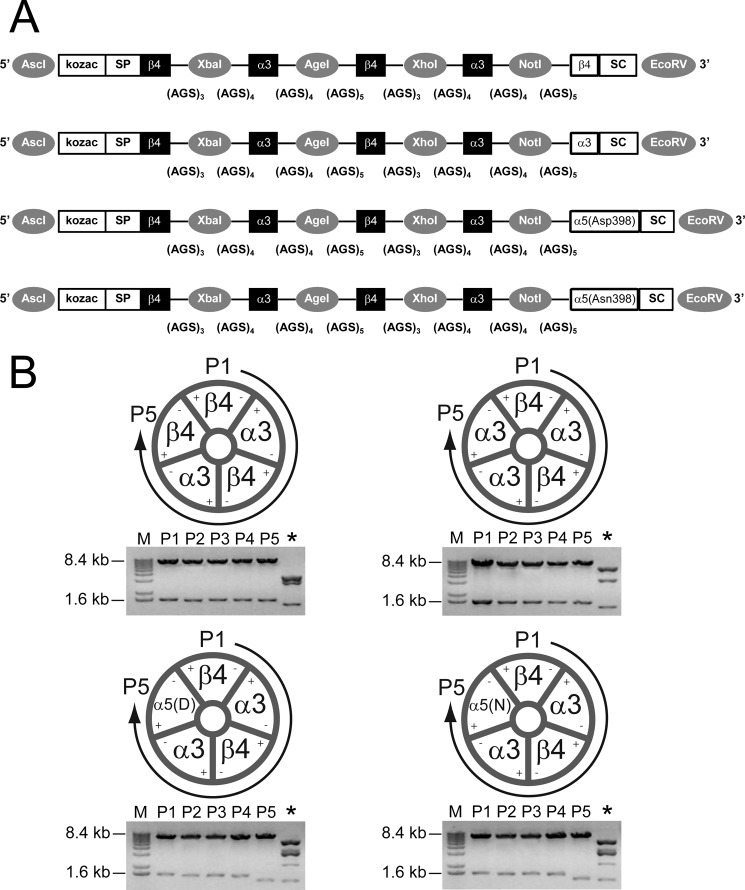
FIGURE 1.
Linked (concatemeric) subunit design of α3β4α5 receptors. A, shown is a schematic illustration of (from top to bottom) β4α3β4α3β4, β4α3β4α3α3, β4α3β4α3α5(Asp-398), and β4α3β4α3α5(Asn-398) constructs. Each construct is flanked with AscI and EcoRV restriction sites (5′ and 3′, respectively; indicated by gray circles) for subcloning into high expression oocyte vectors. Kozac and the β4 signal peptide (SP) were retained only for the 1st position. Flanking each subunit position are unique restriction sites (indicated by gray circles) used in concatemer design (for example, AscI and XbaI used in exchanging nAChR subunits at position 1; XbaI and AgeI sites were used in exchanging nAChR subunits at position 2; etc.). Concatemers varied in composition only at position 5, containing either the β4, α3, or two naturally occurring variants of the α5 nAChR subunit (aspartic acid (Asp-398) or asparagine (Asn-398)). Stop codons (SC) were added at the 3′ end of subunit position 5. The number of AGS repeats flanking each subunit is listed below each linker region. B, shown is stoichiometry of β4α3β4α3β4, β4α3β4α3α3, β4α3β4α3α5(Asp-398), and β4α3β4α3α5(Asn-398) constructs. Concatemers form pentameric receptors by joining the positive interface of the nAChR subunit at position 1 (P1) and the negative interface of position 5 (P5). Restriction digest (below each schematic) using unique restriction sites (as mentioned above) was used to verify each subunit within its respective position (P1-P5). An additional restriction digest (*) using ScaI was performed to diagnose correct subunit composition and order. M, molecular mass markers.