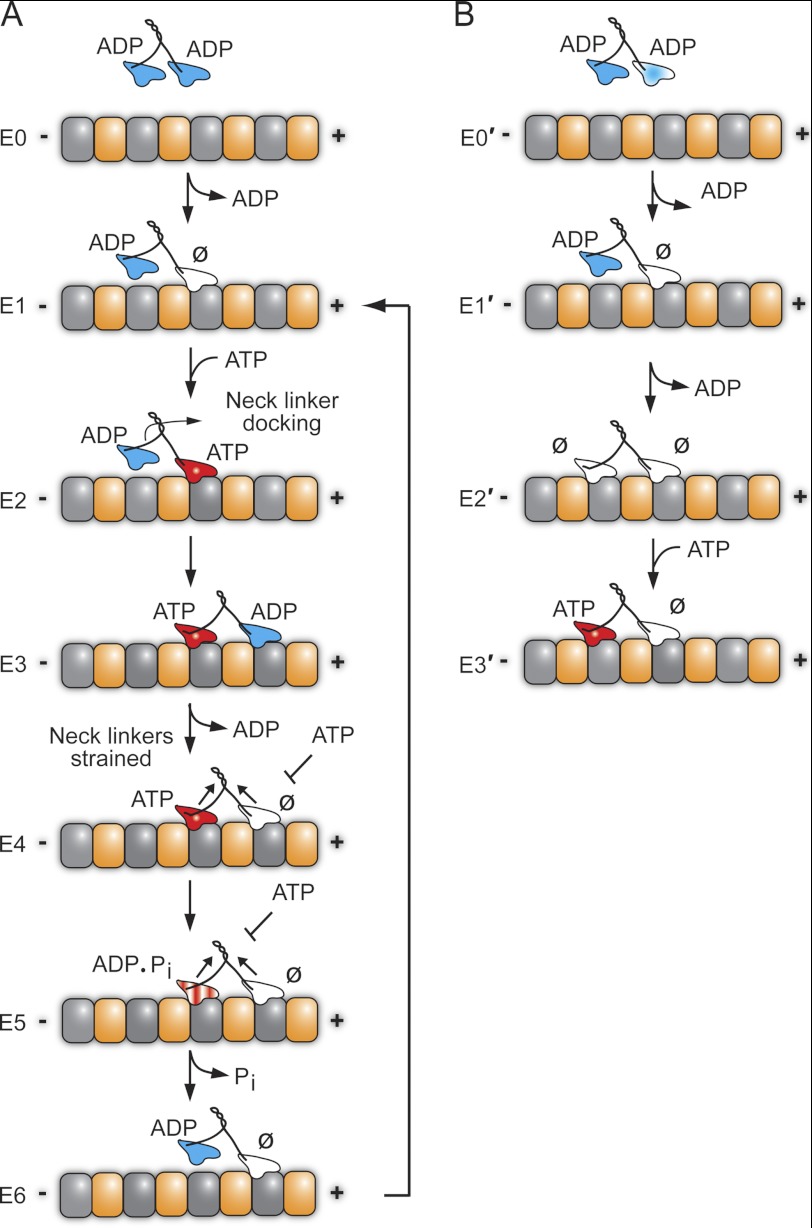
FIGURE 8.
Stepping models for CENP-E. A, CENP-E in solution holds ADP tightly bound at each head. The processive run begins upon MT collision to establish the MT plus-end-directed orientation of CENP-E (E1 state) in which the leading head is tightly bound to the MT and nucleotide-free with the trailing head detached with ADP tightly bound. ATP binding at the leading head triggers a series of structural transitions including neck linker docking that advances the ADP-bound head 16 nm forward to the next MT binding site (E2–E3). ADP release is fast, resulting in the E4 two-head-bound state. Strain develops between the two heads such that ATP cannot bind to the leading head (E4). ATP hydrolysis followed by phosphate (Pi) release and detachment of the ADP trailing head relieve the interhead tension (E4–E6). The leading head can now bind ATP, and the cycle is repeated for another 8-nm step coupled to one ATP turnover. B, alternative model for the entry of CENP-E into a processive run. This model proposes that CENP-E enters the processive run from the E2′ state in which both heads are tightly bound to the MT and nucleotide-free as observed for kinesin-5 Eg5. The MT plus (+) and minus (−) ends are indicated with α-tubulin in gold and β-tubulin in gray. ∅, nucleotide-free.