FIGURE 3.
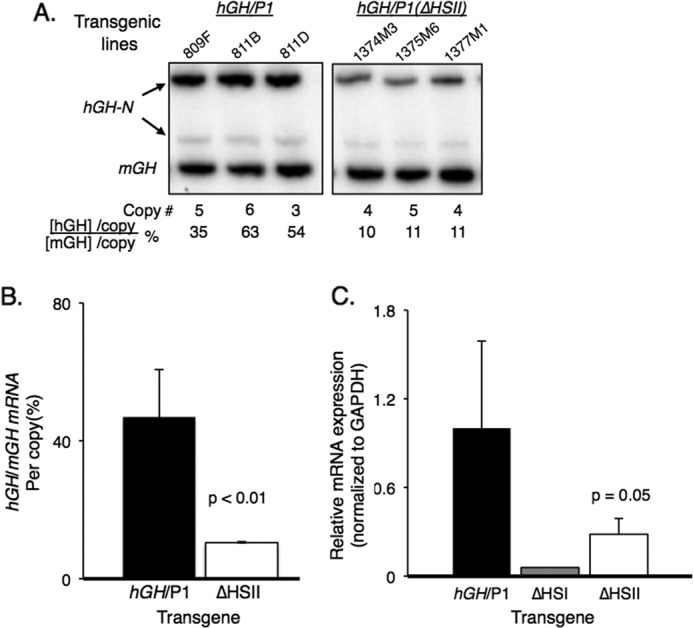
Deletion of HSII from the hGH/P1 transgene resulted in significant loss of hGH-N transgene expression. A, RT-PCR analysis of hGH-N transgene expression. Total RNA was extracted from the pituitaries of three hGH/P1 and three hGH/P1(ΔHSII) transgenic mice. Each RNA sample was reverse transcribed to generate a cDNA library. A PCR was conducted with 32P-radiolabeled primers that co-amplify human and mouse GH mRNAs. The amplified cDNA products were digested with BstNI to separate mouse from human products, and the samples were visualized on an acrylamide gel. The mouse- and human-specific GH cDNA fragments are labeled. The faint band represents an alternatively spliced hGH-N transcript that was not included in the quantification. Transgene copy numbers for each mouse line and the normalized percent hGH-N expression are shown below the autoradiogram. B, quantification of hGH-N transgene expression by co-RT-PCR. All three hGH/P1(ΔHSII) transgenic lines were analyzed in parallel with four hGH/P1 lines. A minimum of seven mice carrying each line was assayed. Error bars indicate 1 S.D. The difference between the products from each construct was significant; p < 0.01 (Student's t test, two-tailed). C, quantification of hGH-N transgene expression by quantitative PCR. Quantitative PCR was conducted on RNA isolated from pituitaries of three hGH/P1(ΔHSII) and three hGH/P1 transgenic mice. A single sample from an hGH/P1(ΔHSI) mouse was included for an additional comparison. The reduced expression in the ΔHSII animals was significant; p = 0.05 (Student's t test, two-tailed).
