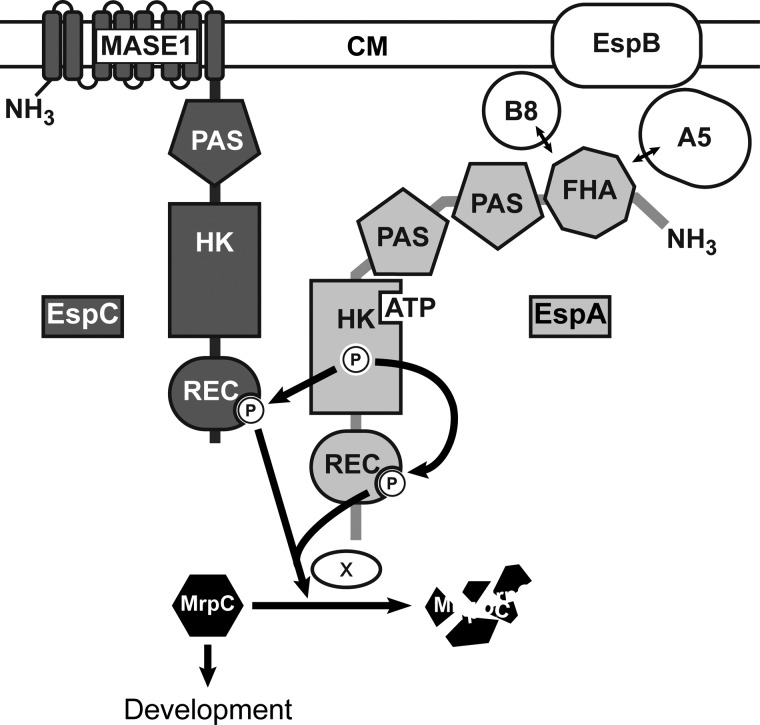
FIGURE 8.
Model of the Esp signaling system. Two-hybrid histidine kinases EspC (dark gray) and EspA (light gray) regulate the accumulation rate of an important developmental regulator, MrpC, to ensure appropriate and coordinated progression through the M. xanthus developmental program. The combined phosphorylation of EspA and EspC receiver domains (REC) activates an unknown protease or protease targeting factor (×) to stimulate MrpC turnover. EspA histidine kinase region (HK) autophosphorylates and donates a phosphoryl group to both its own and EspC receiver domains. EspA may also act as a phosphatase on EspA and or EspC REC. Autophosphorylation of EspC histidine kinase domain is not required for stimulating MrpC turnover under laboratory developmental conditions. EspA activity is controlled by a signaling module consisting of two serine/threonine kinases, PktA5 (A5) and PktB8 (B8), and a putative transport protein (EspB) predicted to reside in the cytoplasmic membrane (CM) (37, 41). PktA5 and PktB8 are thought to interact with the Forkhead associated (FHA) domain in EspA (41). Two PAS domains in EspA and one in EspC may be involved in sensing internal or membrane-associated redox stimuli (65). EspC is predicted to be anchored in the CM by putative MASE1 sensing domain of unknown function (64). The array of signaling domains may allow cell fate-specific accumulation of MrpC within the developmental program.