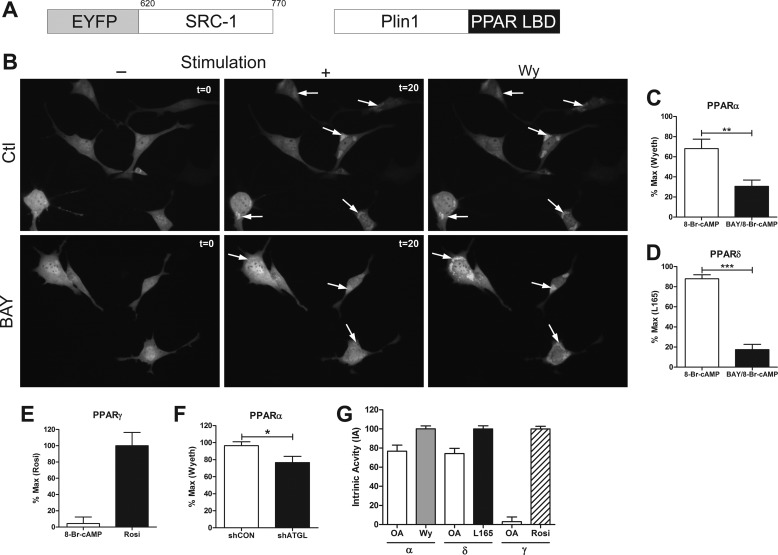
FIGURE 6.
Ligands for PPARα and -δ, but not PPARγ, are created at the lipid droplet surface in response to lipolysis. A, schematic representation of constructs used for the fluorescent reporter assays (amino acids for SRC1 are shown). Plin1, Perilipin-1; LBD, ligand-binding domain. B, brown adipocytes transfected with a PPARα fluorescent reporter were pretreated with DMSO (Ctl) or BAY (5 μm) for 10 min. Representative images shown prior to stimulation with 1 mm 8-Br-cAMP (−; t = 0) or after 20 min of stimulation (+; t = 20) and after the addition of PPARα ligand, Wy (100 μm). C, the PPARα reporter was quantified by normalizing the region of interest after treatment with 8-Br-cAMP or BAY/8-Br-cAMP to the maximal effect of Wy-14,263 (Wyeth) from 2–3 coverslips/experiment (n = 3). D, the PPARδ reporter was normalized to the maximal effect of L165 (10 μm). The effect of BAY was determine by unpaired t test (***, p < 0.001; **, p < 0.01). E, the PPARγ reporter was quantified by normalizing the region of interest after 8-Br-cAMP to the maximal effect of rosiglitazone (Rosi, 10 μm). F, the PPARα reporter was quantified in shCON and shATGL brown adipocytes after treatment with 8-Br-cAMP as above, and the difference was determine by unpaired t test (*, p < 0.05). G, brown adipocytes transfected with reporters for PPARα, -δ, and -γ were treated with 400 μm oleic acid (OA), and the data were normalized to the intrinsic activity (IA) of the respective ligands, Wy, L165, and rosiglitazone.