Figure 4.
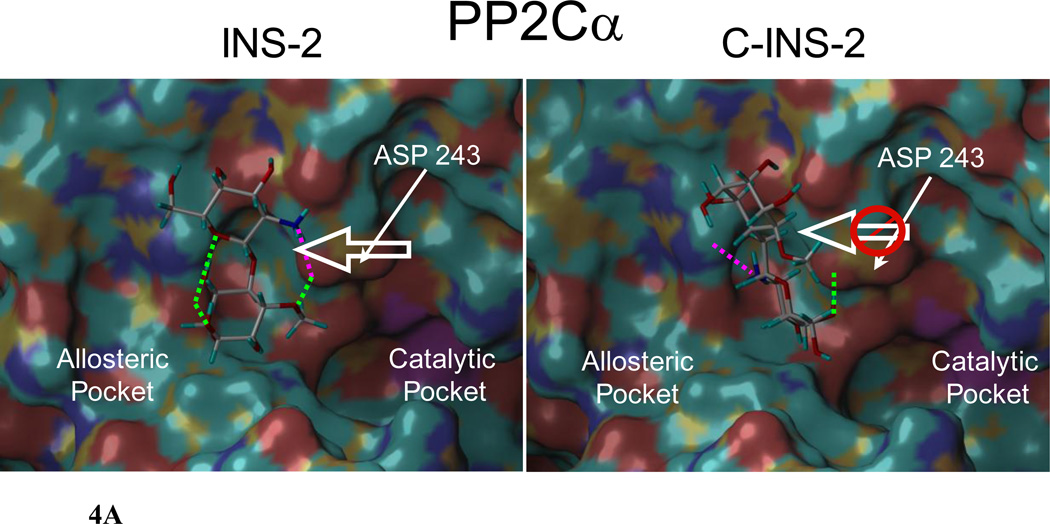
A. INS-2 and C-INS-2 docked in silico into the allosteric site of PP2Cα. H-bonds to the amino group indicated in pink. For the enzyme to be fully active Asp 243 moves to open up the catalytic site. Note the significant difference in position of Asp 243 with the two glycans. INS-2 pulls the acidic residue Asp 243 toward the allosteric pocket and away from the catalytic site favoring hydrolysis. C-INS-2 still binds to this region, but does not pull Asp 243 towards the allosteric pocket, and is inactive.
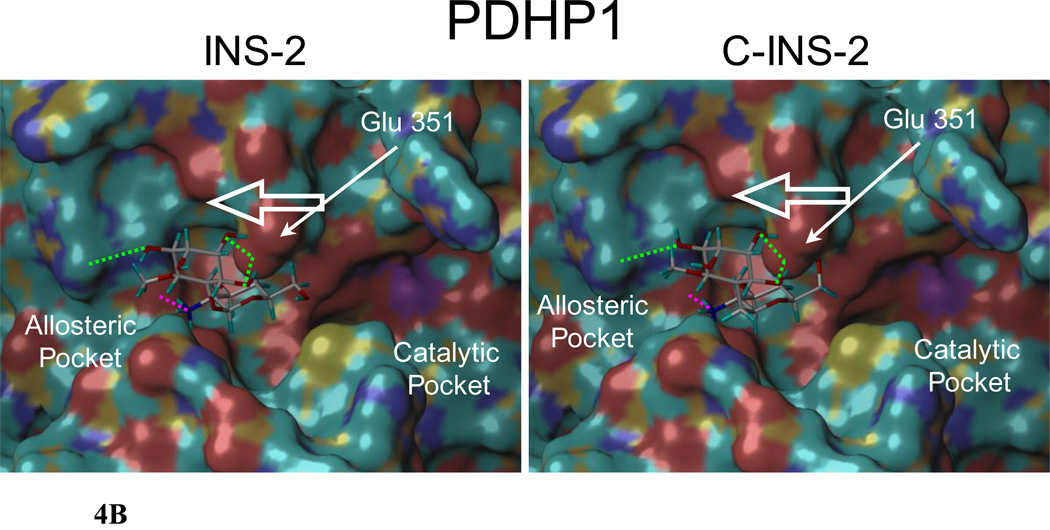
B. INS-2 and C-INS-2 docked in silico into the allosteric site of PDHP1. H-bonds to the amino group indicated in pink. Both are similarly positioned to pull the acidic residue Glu 351 towards the allosteric pocket and away from the catalytic site favoring hydrolysis.