Fig. 3.
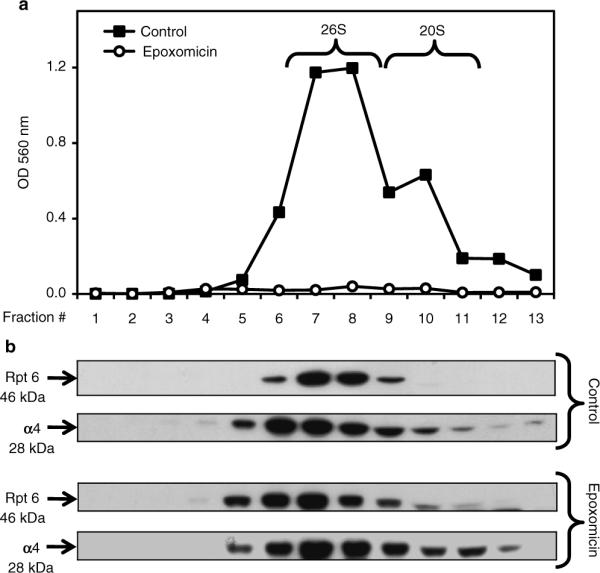
Sedimentation velocity of proteasomes in SK-N-SH cells. Cells were treated for 24 h with DMSO (control, vehicle) or epoxomicin (25 nM). Total lysates (2 mg protein/sample) were fractionated by glycerol density gradient centrifugation (10–40% glycerol corresponding to fractions 13 to 1). (a) Aliquots (50 μl) of each fraction obtained from control (black squares)- and epoxomicin (white circles)-treated cells were assayed for chymotrypsin-like activity with Suc-LLVY-AMC. (b) Immunoblot analyses of each fraction probed with antibodies that react with the proteasome (α4, core particle; Rpt6, 19S regulatory particle). Proteins were precipitated with acetone from 450 μl of each fraction. The fractions were obtained from control- and epoxomicin-treated cells.
