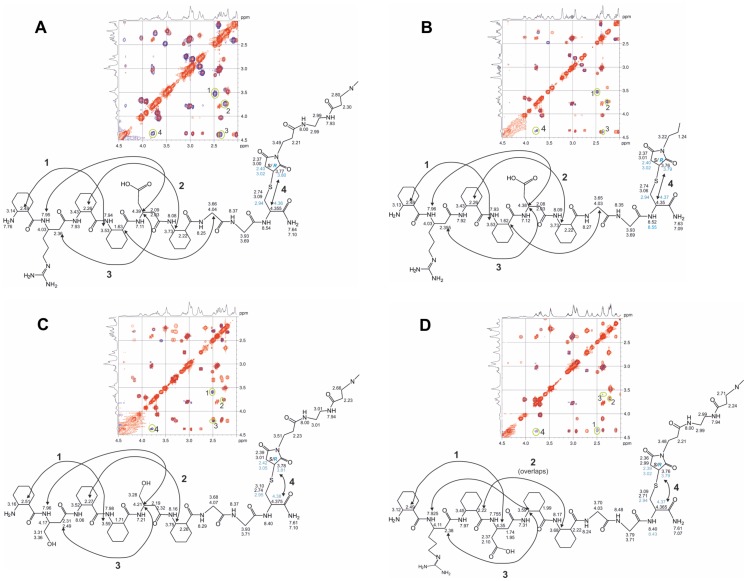
Figure 4. NMR assignments and long-range NOE interactions.
Data are displayed for the foldamer segments and the maleimide diastereomers for 7, 8, 9 and 10 in panels A, B, C and D, respectively. Crosspeaks in the overlaid TOCSY (red) and ROESY (blue) spectra prove the H14 structure of the foldamers. The long-range NOEs supporting the helical conformation were observed in aqueous medium. The addition of the thiol group to the maleimido moiety generates an additional stereogenic center. The NMR resonances of the Cys-maleimide linker region are split and their integrals indicate that the addition is not stereoselective; R and S configurations can be found in equimolar ratio (S and R maleimide diastereomers are signed with black and blue, respectively). Since this undetermined configuration moiety is in the flexible part of the molecule, the effect of the chiral center does not propagate further toward either the foldamer part or the PAMAM template.