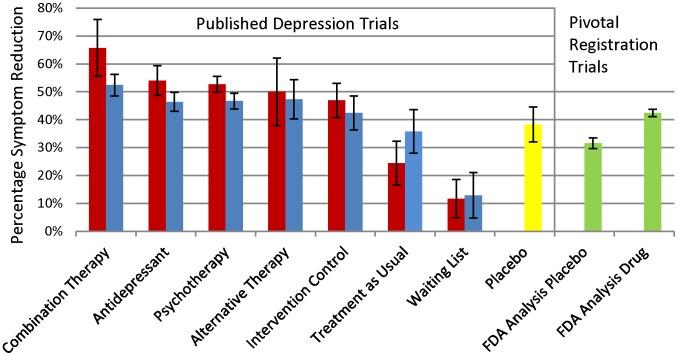
Figure 2. Mean Percentage Symptom Reduction from Un-blinded and Blinded Treatment Arms from Published Depression Trials Compared to Data from Pivotal Registration Depression Trials as Reported by the FDA.
Red Bars Represent Un-Blinded Trial Arms Blue Bars Represent Blinded Trial Arms Yellow Represents Placebo Control Arms from Published Non-Registration trials Green Bars Represent Data from Pivotal Registration Trials The mean percentage symptom reduction was weighted by the number of assigned patients. Error Bars Represent 95% Confidence Intervals. Active treatment arms consist of combination antidepressant + therapy, antidepressants, psychotherapy, antidepressant therapy and alternative therapy. Control treatment arms consisted of placebo control, active intervention control, treatment-as-usual and waiting-list control. Blinded trials were operationally defined as those that utilized depression symptom raters that were blinded to treatment assignment of the patients.