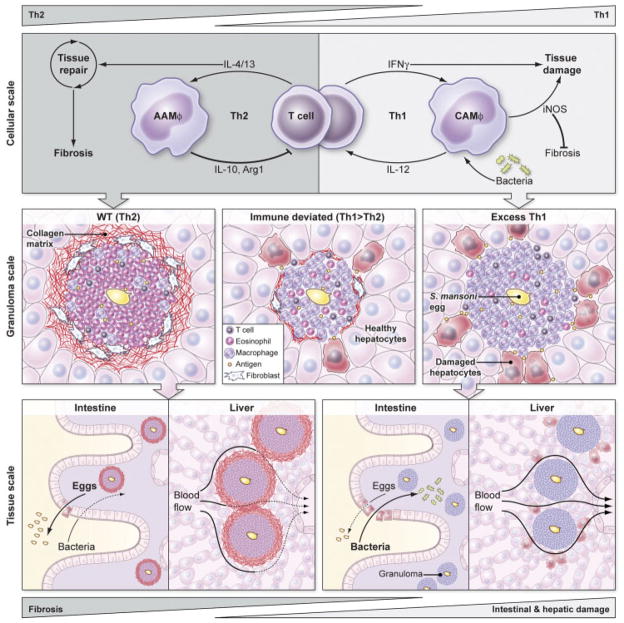
Figure 1.
A model of how macrophage activation regulates the pathogenesis of schistosomiasis at the scale of cells, granulomas, and tissues. In the WT response to S. mansoni (left), IL-4 and IL-13 from Th2 cells promote tissue repair, alternatively activate macrophages, and engage negative feedback loops by inducing AAMϕs to produce IL-10, Arg1, and other factors that limit T-cell proliferation and activation. This WT Th2 response creates eosinophil-rich granulomas around schistosome eggs surrounded by a collagen matrix deposited by IL-4/13-activated fibroblasts that protects the surrounding hepatocytes and other parenchymal cells from toxic egg-derived antigens. Normal granulomatous inflammation and AAMϕs enable schistosome eggs to cross from the mesenteric blood, through the intestine, and into the lumen while promoting wound healing, which maintains sufficient barrier integrity to shield the host from enteric bacteria. However, the accumulation of granulomas causes Th2-dependent fibrosis and collagen deposition that ultimately impedes blood flow through the liver, creating portal hypertension and dilated collateral vessels that are prone to rupture. Exaggerated Th2 immunity accelerates and exacerbates liver scarring but does not seem to significantly harm hepatocytes or disrupt liver function in the short term. If an aberrant Th1 response develops against S. mansoni eggs (right), IFN-γ classically activates macrophages that express IL-12 and iNOS, generating a positive feedback loop that can damage surrounding tissues. The excessive Th1 response generates granulomas with few eosinophils, numerous CAMϕs, and fails to activate fibroblasts to synthesize the collagen matrix which helps to contain egg antigens and protect surrounding parenchymal cells. Th1 granulomas and CAMϕs also appear to be less effective than AAMϕs at wound healing and at facilitating the transit of eggs through the wall of the intestine, leading to breaks in the epithelial barrier that cause septicemia as increased numbers of bacteria from the lumen penetrate the breach. Th1-dependent immunity induces minimal liver fibrosis and is assumed not to significantly interrupt blood flow or cause portal hypertension. Immune deviation in schistosomiasis, by vaccinating hosts with S. mansoni eggs plus IL-12, for example, can reduce both Th2- and Th1-dependent pathologies (center). A slight bias towards a Th1 response contributes to the development of more classically than alternatively activated macrophages, resulting in smaller granulomas and reduced collagen deposition via an iNOS-dependent mechanism. The reduced but still present AAMϕs and Th2 response also limits hepatocyte injury and helps preserve intestinal integrity. Thus, a mixed Th1/Th2 response or slightly biased Th1 responses appear to provide the greatest degree of protection during chronic S. mansoni infection by minimizing fibrosis while simultaneously protecting the host from intestinal and hepatic damage caused by S. mansoni eggs.