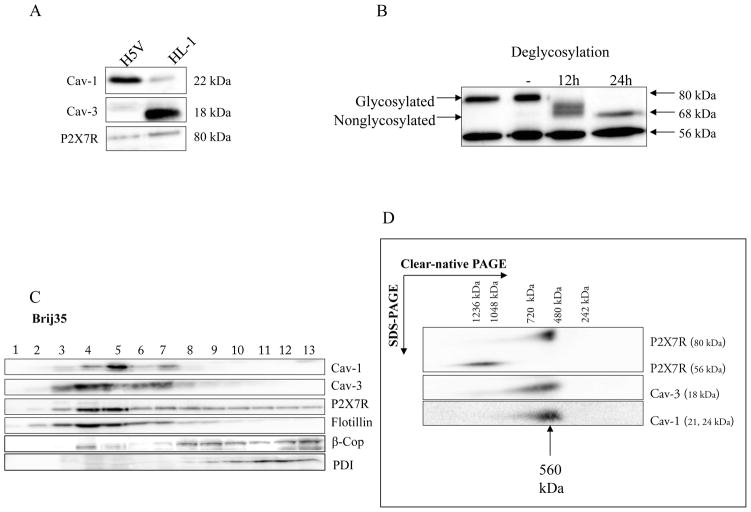
Fig. 5. Biochemical analysis of immortal cell lines.
A Analysis of Cav-1, Cav-3 and P2X7R expression in H5V and HL-1 cells.
Cav-1, Cav-3 and P2X7R expression was measured in cell lysates of H5V and HL-1 cells by SDS-PAGE and Western blot analysis. 50 #g of protein from each sample was loaded on the gel. Western blot analysis was performed using rabbit anti-P2X7R, anti-Cav-1, anti-Cav-3 and anti-γ-tubulin antibody. γ-tubulin served as a loading control. Representative data from three separate experiments are shown.
B P2X7 receptors are glycosylated in HL-1 cells.
Membranes were incubated in the precence of N-glycosidase F for 12 h and 24 h and compared with nontreated membranes (-). Full deglycosylation of the 80 kDa band was indicated by a reduction in molecular mass of approximately 12 kDa to 68 kDa. The mass of the 56 kDa band was unaffected by deglycosylation. Representative data from three separate experiments are shown.
C Characterization of membrane fractions prepared by Brij35 from HL-1 cells.
HL-1 cells were homogenized in a buffer containing 1% Brij35 and subjected to sucrose density gradient centrifugation. Thirteen fractions were collected and an aliquot of each fraction (20 #l) was resolved by SDS-PAGE and subjected to Western blot analysis with antibodies against Cav-1, Cav-3, P2X7R, PDI, β-Cop and flotillin-1. As expected, Cav-1, Cav-3 and flotillin-1 were enriched in fractions 3–7, representing caveolae-enriched membrane fractions. Representative data from three separate experiments are shown.
D Supramolecular organization of P2X7R, Cav-1 and Cav-3 in HL-1 cells.
Native membrane extracts from HL1- cells were isolated and solubilized with digitonin. Proteins were separated by hrCN-PAGE/SDS-PAGE, blotted and probed with antibodies against P2X7R, Cav-1 and Cav-3. Sizes of the molecular mass standards are indicated.