Figure 1.
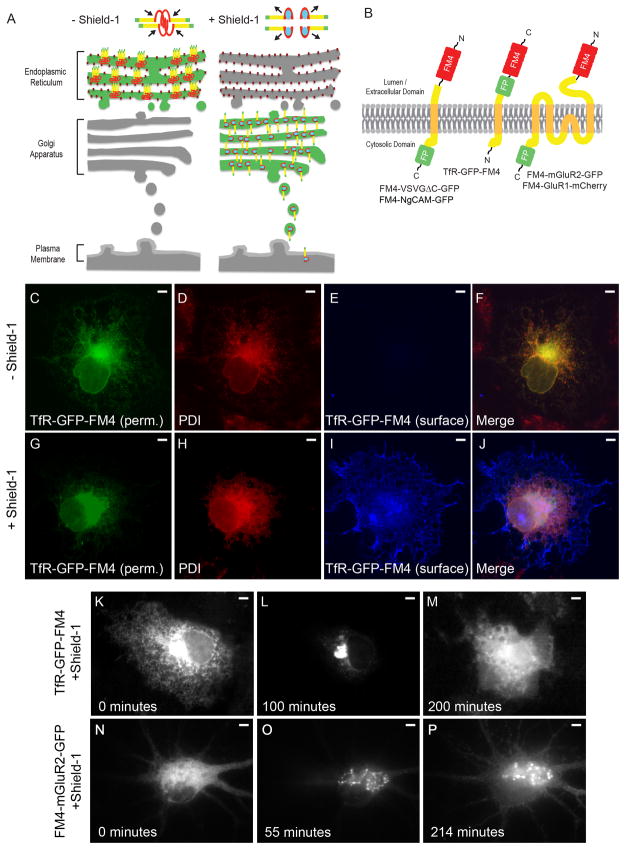
An FM4/Shield-1-based pulse chase system allows for synchronization of transmembrane proteins in the secretory pathway. (A) In the absence of Shield-1 transmembrane proteins (yellow) fused to FM4 domains (red) and to fluorescent proteins (green) multimerize causing clustering of the expressed fusion protein and retention within the ER. Binding of Shield-1 (cyan) to the FM4 domain causes disaggregation of the expressed transmembrane fusion protein, releasing it from the ER and allowing it to proceed through the secretory pathway. (B) Constructs contain 4 FM domains in tandem on the extracellular domain and a fluorescent protein fused to transmembrane proteins. (C, F) Following expression in COS cells and without exposure to Shield-1, TfR-GFP-FM4 (green) localizes in a reticular pattern that colocalizes with the endogenous ER markerPDI (red, D, F). A lack of TfR-GFP-FM4 on the surface (blue, E, F) is consistent with it being retained within the ER. In contrast, in cells exposed to Shield-1 TfR-GFP-FM4 (G, J) only partially colocalizes with PDI (H, J ) and TfR-GFP-FM4 expresses on the cell surface (I, J). Live imaging confirms that when TfR-GFP-FM4 is expressed in a COS cell for 12–16 hours it localizes in a reticular manner, suggesting that it is retained in the ER (K). Following addition of Shield-1 it becomes concentrated in a pattern consistent with Golgi localization (L). Subsequently, it localizes in a more diffuse pattern consistent with expression on the cell surface (M). Similarly, when FM4-mGluR2-GFP is expressed in a neuron for 16 hours, it localizes in a reticular manner consistent with retention in the ER (N). After the addition of Shield-1, FM4-mGluR2-GFP localizes in a perinuclear pattern, suggesting it had migrated to the Golgi (O). Afterwards, FM4-mGluR2-GFP shows a diffuse pattern of labeling consistent with presence on the plasma membrane (P). Scale bar is 5 μm.