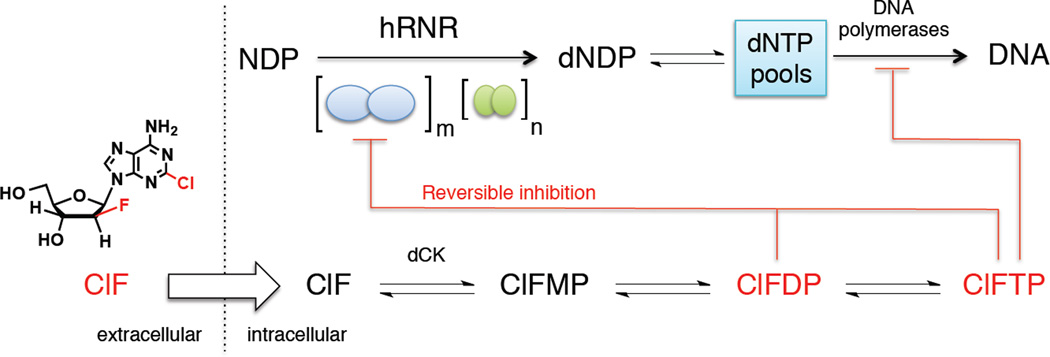
Figure 1. hRNR is a principal target of ClF.
ClF enters the cell by passive diffusion and/or via nucleoside transporters, and is rapidly phosphorylated (Bonate et al., 2006; Zhenchuk et al., 2009). ClFDP and ClFTP both target hRNR (α2)m, resulting in the depletion of dNTP pools including dCTP. Reduction of the latter potentiates production of ClF metabolites by removing negative feedback inhibition of deoxycytidine kinase, dCK, an enzyme that catalyzes monophosphorylation of ClF. Diminution of overall dNTP pool sizes amplifies misincorporation of ClFTP into DNA by DNA polymerase-α and –ε where it functions as a chain terminator. Induction of apoptosis is thought to be ultimately responsible for anticancer activity. ClFTP is a rapid reversible inhibitor of α via binding to the activity (A) site (Ki = 40 nM) and ClFDP is a slow-release reversible inhibitor (Ki* = 17 nM, t1/2= 23 min) of α via binding to the catalytic (C) site, and both cause α hexamerization (Aye and Stubbe, 2011).  and
and  ellipses represent (α2) and (β2 or p53 β2) respectively.
ellipses represent (α2) and (β2 or p53 β2) respectively.