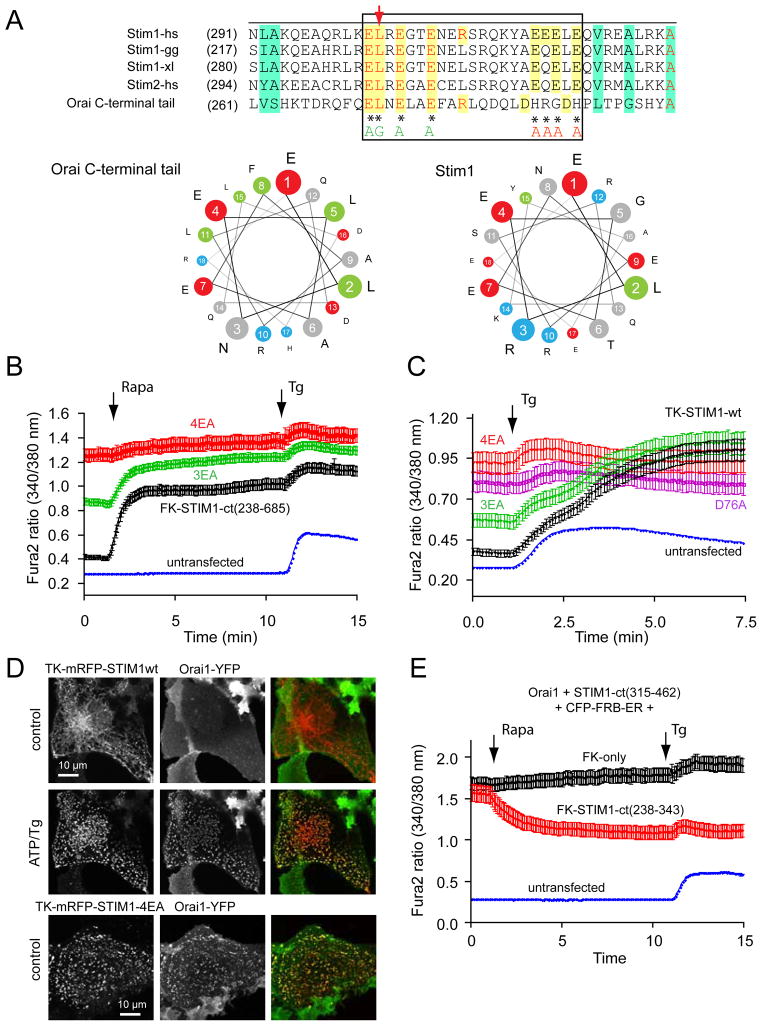
Figure 3.
An acidic segment within the coiled-coil domain of STIM1 is homologous with the Orai1 C-terminal helix and is essential to keep STIM1 in an inactive state. (A) Sequence alignment between STIM1 orthologues and the Orai1 C-terminus identifies a homologous region within the coiled-coil domain. Helical wheel diagram shows the high degree of positional conservation between the Orai1 helix and the proximal part of the acidic STIM1 segment. A Leu to Ser substitution of the residue shown by the red arrow in Orai1 has been shown to prevent its activation by STIM1 (22). Mutations within this segment of STIM1 are labeled by an asterisk (AGAA, or 3EA). Changing only the EL residues in STIM1 (to AG) had negligible effects (not shown). (B) Mutations within this segment (3EA) make the cytoplasmic STIM1 construct partially active without clustering (red). However, mutation of the four E residues in the distal part of the acidic motif (4EA) renders the cytoplasmic STIM1 piece fully constitutively fully active. Means ± S.E.M. are shown (n = 220, 345, 143 and 802 cells for black, green, red and blue traces, respectively obtained in 6–38 separate dishes from 3–9 independent experiment). (C) The same mutations within the full-length STIM1 protein show similar partial or full constitutive activity. For comparison, the effects of the known constitutively active D76A mutant (4) STIM1 is shown. For these experiments the thymidine kinase (TK) driven mRFP-STIM1 constructs were expressed together with untagged Orai1. Means ± S.E.M. are shown (n = 143, 109, 80, 111 and 785 cells for black, green, pink, red and blue traces, respectively, obtained in 3–36 dishes from 3–8 separate experiments). (D) Unlike wild-type STIM1 (upper two rows), the 4EA mutant mRFP-STIM1 forms constitutive clusters together with Orai1-YFP without Ca2+ release from the ER (lower row). This analysis was performed at room temperature. (E) Plasma membrane recruitment of the STIM1 coiled-coil domain containing the inhibitory acidic segment inhibits Orai1-mediated Ca2+ influx activated by the constitutively active STIM1 fragment (315–462). For this experiments COS-7 cells were transfected with the mRFP-fused STIM1(315–462) (non-recruitable!), untagged Orai1 and the ER-recruiter FRB together with either the mRFP-FKBP12-fused STIM1-ct(238–343) [FK-STIM1-ct (238–443), red] or mRFP-FKBPonly (FK-only, black). Rapamycin addition decreased the high basal Ca2+ in a significant fraction of cells (~16 %) in the STIM1-ct(238–343) expressing group while had no effect in any cells in the FKBPonly group. Means ± S.E.M. are shown (n = 119, 80 and 247 cells for black, red and blue traces, respectively, obtained in 5–10 separate experiments).