Table 2.
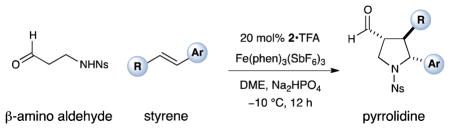
| |||
|---|---|---|---|
| 1 |
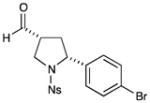 72% yield, 9:1 dr, 93% ee |
2 |
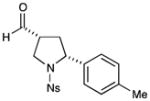 71% yield, 7:1 dr, 91% ee |
| 3 |
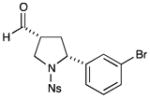 76% yield, 8:1 dr, 93% ee |
4 |
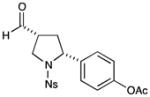 73% yield, 8:1 dr, 92% ee |
| 5 |
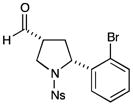 76% yield, 3:1 dr, 94% ee |
6 |
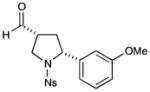 75% yield, 10:1 dr, 89% ee |
| 7 |
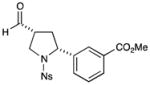 61% yield, 5:1 dr, 89% ee |
8 |
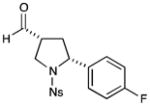 81% yield, 8:1 dr, 91% ee |
| 9c |
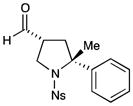 67% yield, 3:1 dr, 92% ee |
10c |
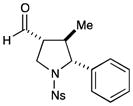 50% yield, >20:1 dr, 96% ee |
Results listed as product, yield, diastereomeric ratio (dr), enantiomeric excess (% ee).
Diastereomeric ratio, % ee determined as in Table 1.
Reaction conducted at −20 °C using THF and Fe(phen)3(PF6)3.
