Table 1.
Lichen compounds and their potency for LF inhibition.
| Compound | Name | Structure | IC50 (µM), MKK6 cleavage |
|---|---|---|---|
| 1 | Stictic acid (StA) | 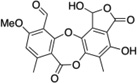 |
24 ± 3 |
| 2 | Salazinic acid | 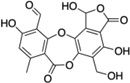 |
42 ± 5 |
| 3 | Parellic acid | 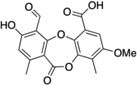 |
125 ± 20 |
| 4 | Gangaleoidin | 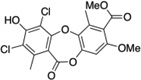 |
>250 |
| 5 | Physodic acid | 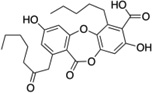 |
>250 |
| 6 | Lecanoric acid | 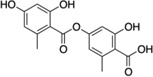 |
No inhibition |
| 7 | Atranorin | 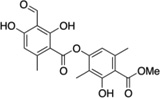 |
No inhibition |
| 8 | Reduced stictic acid | 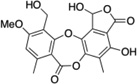 |
>250 |
| 9 | Reduced parellic acid | 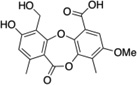 |
>250 |
