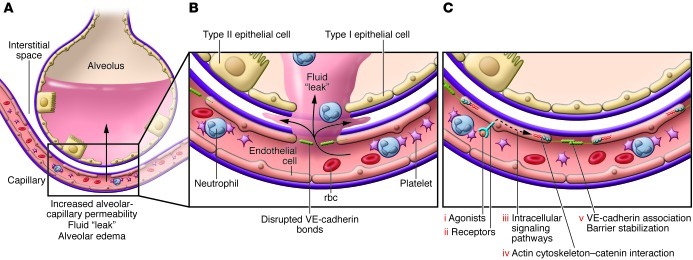
Figure 3. Molecular targets for new therapies that can lead to endothelial and epithelial barrier stabilization and reversal of increased permeability.
(A) Disrupted alveolar barrier function, resulting in increased permeability to water, proteins, and other solutes, is a hallmark of clinical and experimental ALI. Intra-alveolar accumulation of neutrophils, other leukocytes, and erythrocytes is also associated with altered endothelial and epithelial barrier function. TNF-α, IL-1, thrombin, and microbes and their toxins — including LPS, noxious agents, and factors generated by neutrophils and platelet-leukocyte interactions — can destabilize and disrupt alveolar barrier function, leading to increased permeability. (B) Disruption of VE-cadherin bonds is a central mechanism of altered endothelial barrier function in experimental ALI and in models of sepsis and systemic vascular destabilization. VE-cadherin is an endothelial-specific adherens junction protein that mediates Ca2+-dependent homophilic interactions at the lateral cell membranes of adjacent endothelial cells. VE-cadherin is regulated by cytoplasmic associations with catenins and actin and by cytoskeletal organization, in addition to intracellular signaling by Rho and Rac. Disruption of VE-cadherin bonds also facilitates transendothelial migration of leukocytes and, in some studies, is associated with accumulation of leukocytes and platelets in microvessels. (C) Stabilizing agonists (i) or small-molecule mimetics bind to stabilizing receptors (ii) on endothelial cells in alveolar and systemic vessels, restoring barrier integrity. Stabilizing agonists include S1P, Slit2N, Ang1, atrial natriuretic peptide, APC, and ATP; multiple intracellular pathways and mechanisms are implicated (iii) (reviewed in refs. 58, 61, 64). These intracellular mechanisms favorably influence cytoskeletal architecture, preserve catenin–VE-cadherin cytoplasmic interactions, prevent VE-cadherin internalization, and/or promote adherens junction formation (iv and v).