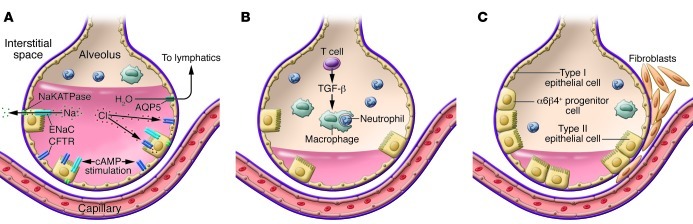
Figure 4. Resolution of ALI requires removal of alveolar edema fluid, removal of the acute inflammatory cells, and repair of the injured alveolar epithelium.
(A) Alveolar edema fluid reabsorption is driven by vectorial transport of sodium and chloride from the airspaces to the lung interstitium, creating a mini–osmotic gradient. Sodium is transported across apical sodium channels (including epithelial sodium channel [ENaC]) and then extruded basolaterally by sodium-potassium ATPase (NaKATPase). Chloride is transported by transcellular or paracellular pathways. In the presence of endogenous or exogenous cAMP stimulation, the rate of alveolar fluid transport increases substantially, accomplished by increased expression and activity of ENaC, NaKATPase, and opening of the CFTR. For net fluid clearance to occur, however, there needs to be a reasonably intact alveolar epithelial barrier (see C). AQP5, aquaporin 5. (B) The resolution of inflammation in ALI and ARDS requires the removal of neutrophils from the distal airspace of the lung. Neutrophils are normally taken up by alveolar macrophages, a process termed efferocytosis. The rate of neutrophil clearance can be accelerated by regulatory T lymphocytes, in part by release of TGF-β. (C) Restoration of the alveolar epithelial barrier initially occurs by reepithelialization of the epithelial surface by alveolar type II cells. Although it was previously thought that this occurred via proliferation of resident type II cells, new work suggests there may be niches of progenitor cells that also contribute. An α6β4+ progenitor cell has been identified in the mouse lung that is responsible for restoration of the alveolar epithelial barrier after bleomycin-induced lung injury (88). Thus, repair may occur by endogenous stem cell proliferation, not just by epithelial cell migration and proliferation of existing differentiated cells.