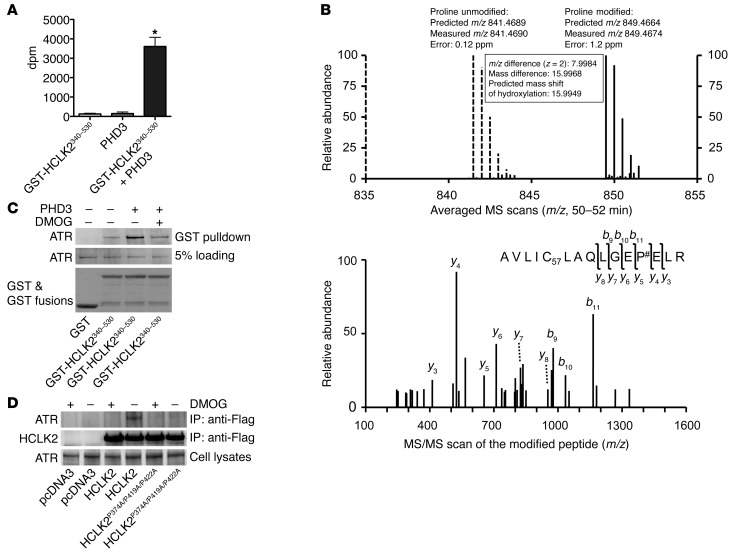
Figure 5. PHD3 hydroxylation of HCLK2 mediates the interaction between HCLK2 and ATR.
(A) In vitro hydroxylation assays using 2-oxo-glutarate [1-14C] as the cosubstrate were performed in the presence of GST-HCLK2340–530 or PHD3 as indicated. The released 14CO2 (mean ± SEM) was trapped and measured. n = 3; *P < 0.01 versus PHD3 alone. (B) Flag-HCLK2340–530 immunoprecipitated from HeLa cells was trypsin digested and analyzed by LC-MS/MS. Modified peptide sequence AVLIC57LAQLGEP#ELR (C57 indicating mass shift of 57.0215 Da by iodoacetamide alkylation on the Cys residue; # indicating proline hydroxylation) was identified based on the high-resolution MS/MS survey scan comparison between the unmodified form (dashed lines) and the hydroxylated form (solid lines) as well as the MS/MS scan of the hydroxylated peptide. The unmodified form was detected as a doubly charged monoisotopic ion of 841.4690 m/z, whereas the modified form was identified as 849.4674 m/z. The modification was further validated by multiple product ions (b and y ions) in the MS/MS scan by collision-induced dissociation. (C) GST or GST-HCLK2340–530 immobilized on GSH beads were incubated with Flag-ATR purified from HEK293 cells, either directly or after 60 minutes of incubation with PHD3 in the presence or absence of DMOG. The pull-down products were analyzed by Western blot analysis. (D) HeLa cells transfected with pcDNA3, Flag-HCLK2, or Flag-HCLK2P374A/P419A/P422A were treated with DMOG for 6 hours. Coimmunoprecipitation and Western blot analysis were then performed with the indicated antibodies. In C and D, representative blots from 2 similar experiments are shown.