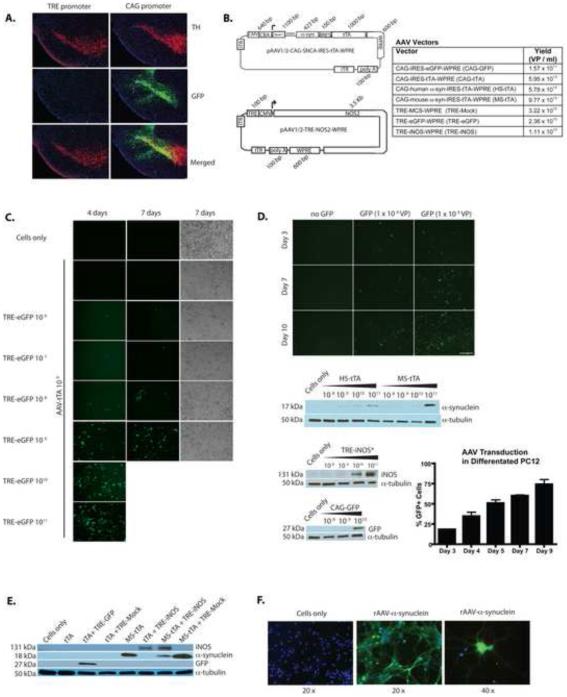
Fig. 1. Expression of rAAV delivered genes and vector design.
(A) Representative 30 μm coronal brain images showing that AAV2/1-TRE-eGFP-WPRE delivered to the mouse SNpc of CaMKIIa-Tg mice (left half of panel) showed no expression in SNpc due to lack of promoter activity, but AAV2/1-CAG-eGFP-WPRE delivery resulted in robust eGFP expression (green) and co-localization with TH+ neurons (red) in the SNpc of WT mice (right half of panel) at 4-weeks post injection. (B) Design of vectors used to circumvent Tg mouse requirement. Table indicates typical yields [viral particles (VP)] of vectors produced. (C) Titration of TRE-eGFP in 293 cells (co-transduced with AAV2/1-CMV-tTA-WPRE at 109 VP/ 2 × 105 cells) showing increasing expression with increasing infective dose. (D) TRE-eGFP transduced 293 cells (co-transduced with AAV2/1-CMV-tTA-WPRE at 109 VP/ 2 × 105 cells) show increased eGFP expression over the course of 10 days. Western blots show expression of HS-tTA, MS-tTA, and CAG-eGFP delivered genes resulting in dose dependent protein expression at 5 days post transduction in 293 cells. *Indicates cells were also transduced with AAV2/1-CAG-IRES-tTA-WPRE at 1010 VP/ 2 ×105 cells. Graph shows percent of cells visibly expressing GFP on days after treatment with AAV-GFP (TRE-eGFP) in differentiated PC12 neurons (co-infected with AAV2/1-CAG-IRES-tTA-WPRE). Data represent mean percentages (± SEM) using fluorescence and light microscopy images from 3 separate wells of cells (r2 = 0.93). (E) AAV delivered genes show robust expression in primary cortical neurons from embryonic day 18 (E18) mice when used alone or in combination by western blot. (F) Immunofluorescence staining in primary neuron cultures showing expression and distribution of α-syn 12 days post-transduction with MS-tTA. Data are representative of 3 independent experiments.