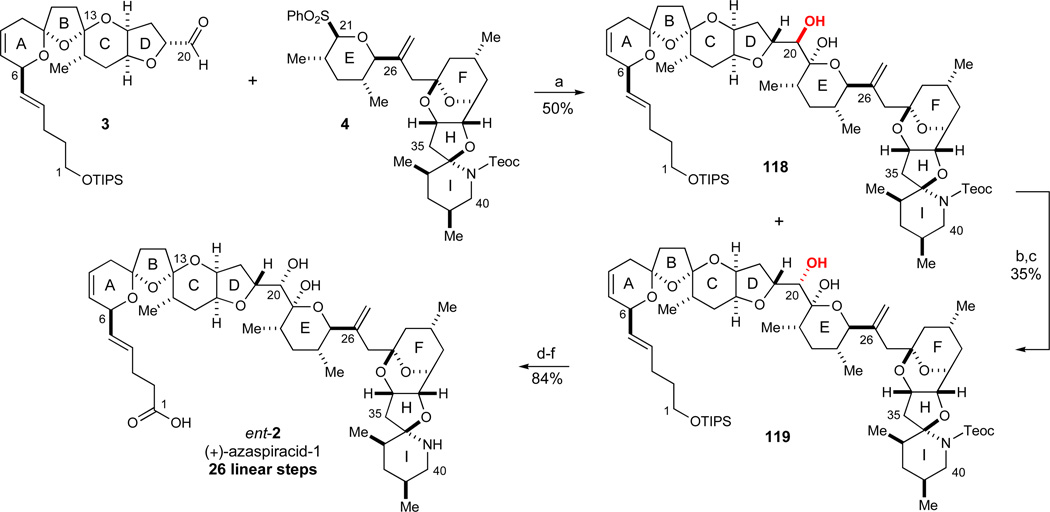
Scheme 21.
Final fragment coupling and completion of the synthesis. Reagents and conditions: a) 4 (2.2 equiv), n-BuLi, −78 °C, then 3 (1.0 equiv), then NaOAc/AcOH buffer, −78 °C to RT, 50% (27% 118, 23% 119); b) (COCl)2, DMSO, Et3N, CH2Cl2, −78 to −20 °C, 63%; c) LiBH4(THF), CH2Cl2, −40 °C, dr > 20:1, 56%; d) TBAF, THF, 0 °C, 93%; e) Dess-Martin periodinane, CH2Cl2, 0 °C; f) NaClO2, NaH2PO4·H2O, 2-methyl-2-butene, t-BuOH, 90% (2 steps). See ref31 for abbreviations