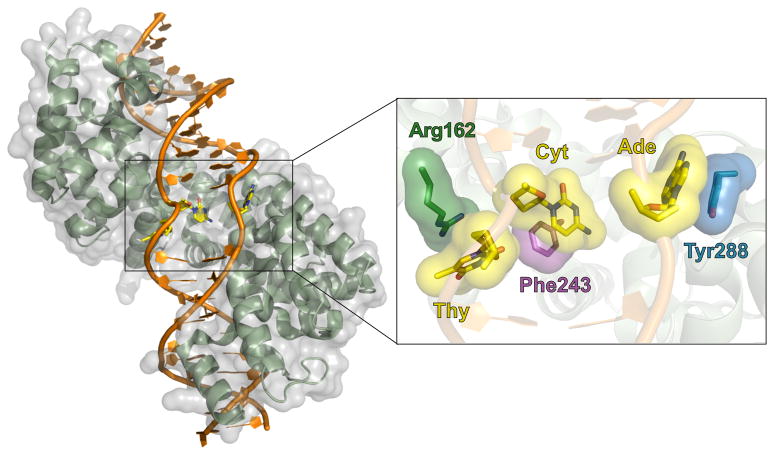
Figure 2. The X-ray Crystallographic Structure of the Mitochondrial Transcription Termination Factor MTERF1 Bound to the tRNALeu Mitochondrial DNA Termination Sequence.
MTERF1 utilizes a unique DNA binding mode that results in duplex unwinding and eversion of three nucleotide bases. MTERF1 is shown in green with its molecular surface shown in transparent grey; the DNA termination site is shown in orange; everted bases are shown as yellow sticks. Inset: π-stacking interactions with the MTERF1 residues (dark green, purple, and blue) stabilize the three corresponding DNA bases (yellow) in an extrahelical position. These interactions are essential for termination activity.