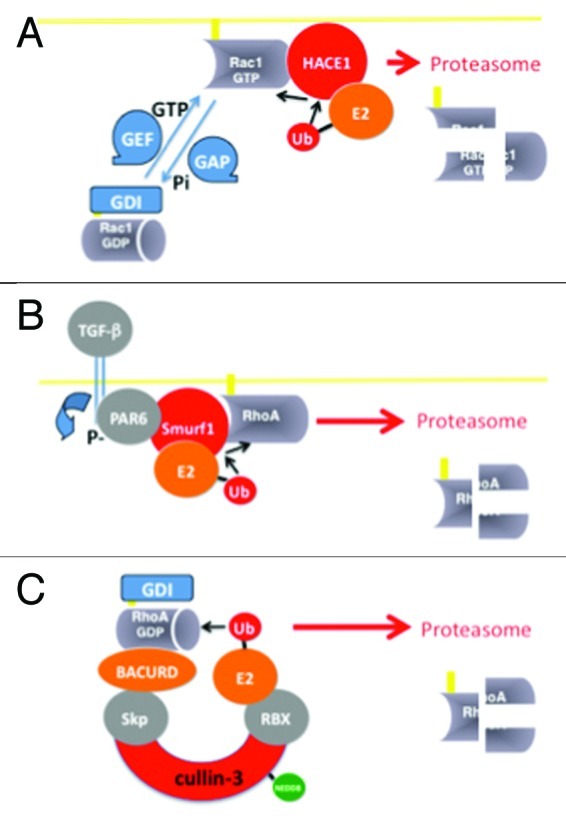
Figure 1. Rho protein ubiquitylation. (A) HACE1 HECT-domain containing protein E3 ubiquitin-ligase binds preferentially to the GTP-bound form of Rac1, and to an ubiquitin conjugated to an E2 enzyme. Ubiquitin molecules are next transferred to a conserved cysteine residue (C876 of HACE1) of the HECT-domain prior to conjugation on Rac1 to form K48-poly-ubiquitin chain, a signal for targeting to the proteasomal machinery. (B) RhoA is a target of Smurf1 HECT-domain containing E3 ubiquitin-ligase. (C) The GDP-bound form of RhoA is recognized by a multi-subunit E3 ubiquitin-ligase homologous to SCF (for Skp1-Cullin-1 and RING-finger), which contains Cullin-3, as scaffold protein, and the BTB-domain containing protein BACURD for specific binding to RhoA. This allows a direct conjugation of ubiquitin to RhoA for subsequent proteasomal destruction.
