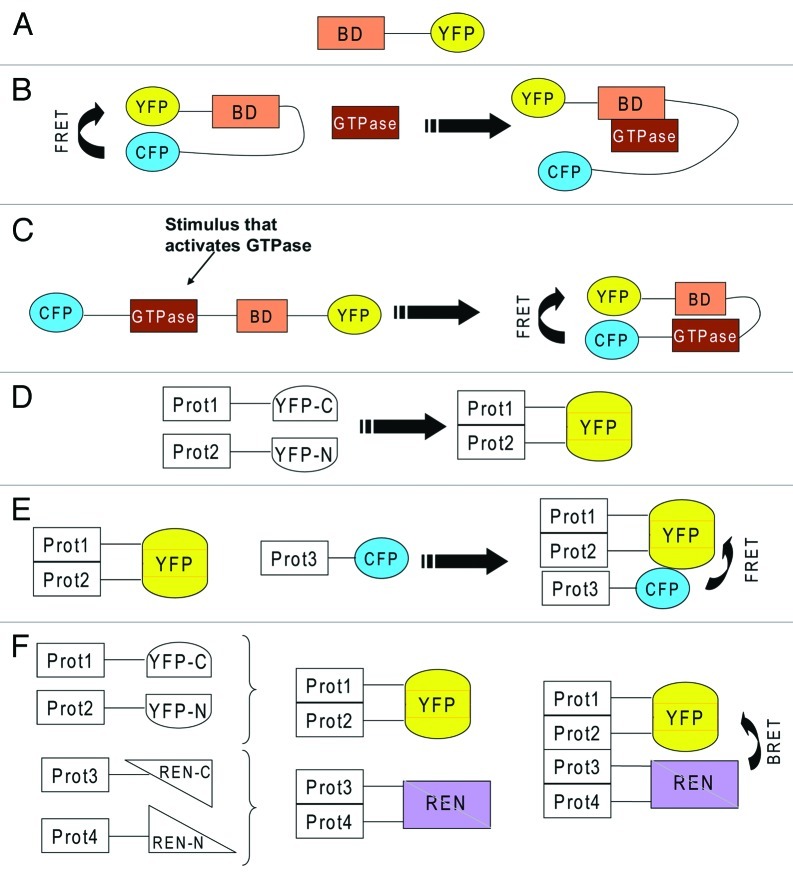
Figure 2. (A) YFP fused to a binding domain (BD) that recognizes an active GTPase. Because of the binding domain, if taken from an effector of a small GTPase, this reporter will specifically bind a certain type of GTPase. (B) BD sandwiched between CFP and YFP, which exhibit FRET because they are in close proximity. An incoming active GTPase squeezes in to bind to BD and displaces CFP and YFP, which leads to loss of FRET. (C) the GTPase and a BD from its effector are sandwiched between CFP and YFP. Activation of the GTPase will lead to binding of BD to the active GTPase which leads to closure of the reporter which allows FRET between CFP and YFP. (D) two proteins (Prot1 &2) are fused to two severed halves of YFP (YFP-C and YFP-N). Interaction of the two proteins will lead to complementation of YFP, which starts to fluoresce. (E) the interaction of two proteins is detected via YFP complementation. A third protein (Prot3) also joins the complex and this is visualized by FRET between CFP and YFP. (F) the interaction of two proteins (Prot 3&4) is detected via YFP complementation. The interaction of two further proteins (Prot 3&4) is detected via complementation of two severed halves of renilla luciferase (REN). The formation of a tetrameric complex is visualized by BRET.

An official website of the United States government
Here's how you know
Official websites use .gov
A
.gov website belongs to an official
government organization in the United States.
Secure .gov websites use HTTPS
A lock (
) or https:// means you've safely
connected to the .gov website. Share sensitive
information only on official, secure websites.