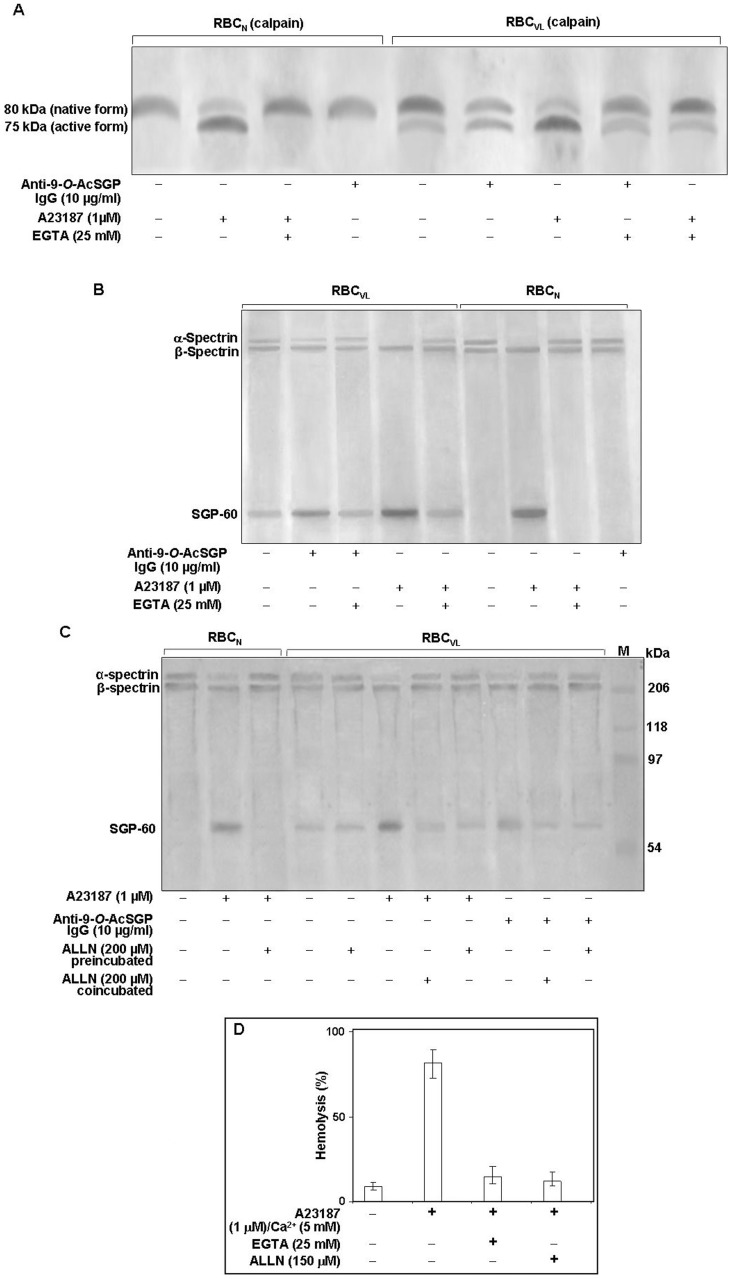
Figure 4. Activation of calpain I in RBCVL or RBCN.
A. Cells (2×107) were incubated with or without anti-9-O-AcSGP IgGVL, anti-9-O-AcSGP IgGNHS (10 µg/ml) or the Ca2+ ionophore A23187 in the absence or presence of EGTA (25 mM) for 60 min at 37°C in Ringer solution, washed with same buffer and lysed by sonication on ice. After removal of cell debris by centrifugation the lysates were separated by SDS-PAGE, and calpain I was detected by Western blot analysis with an anti-calpain-I antibody. B. Enhanced degradation of α-spectrin in sensitized RBCVL. RBCVL and RBCN were suspended separately in Ringer solution and sensitized with anti-9-O-AcSGP IgGVL, anti-9-O-AcSGP IgGNHS or A23187 in the absence or presence of EGTA for 30 min at 37°C. The cells were then lysed as before and the lysates subjected to SDS-PAGE and Western blot analysis with a spectrin-specific antibody. C. Involvement of calpain I in the degradation of spectrin in RBCVL. RBCVL and RBCN were pre-incubated with the calpain I inhibitor ALLN in Ringer solution for 30 min at 37°C before sensitization or co-incubated together with anti-9-O-AcSGP IgGVL or A23187 as positive controls. The cells were processed as before and spectrin detected by Western blot after SDS-PAGE of the cellulaproteins. D. Dependence of the hemolysis of RBCN on the activation of calpain I. RBCN were incubated without or with A23187 and with A23187 plus EGTA or ALLN for 1 h at 37°C, and the degree of hemolysis determined spetrophotometrically as in Figure 1. The results are shown as representative bar graphs of three independent experiments.